JAN 28, 2019
Allergan
Comprehensive Ophthalmology, Retina/Vitreous, Uveitis
Due to the possibility of silicone contamination, Allergan has issued an “urgent drug recall” of their dexamethasone implant, Ozurdex.
In a letter to physicians dated Dec. 28, 2018, the company noted that a silicone particle of approximately 300 microns in diameter may detach from the needle sleeve during administration, possibly delivering the particle into the eye along with the implant. Potential reactions include mild transient visual disturbances, intraocular inflammation and a remote possibility of corneal reaction if the particle migrates into the anterior chamber.
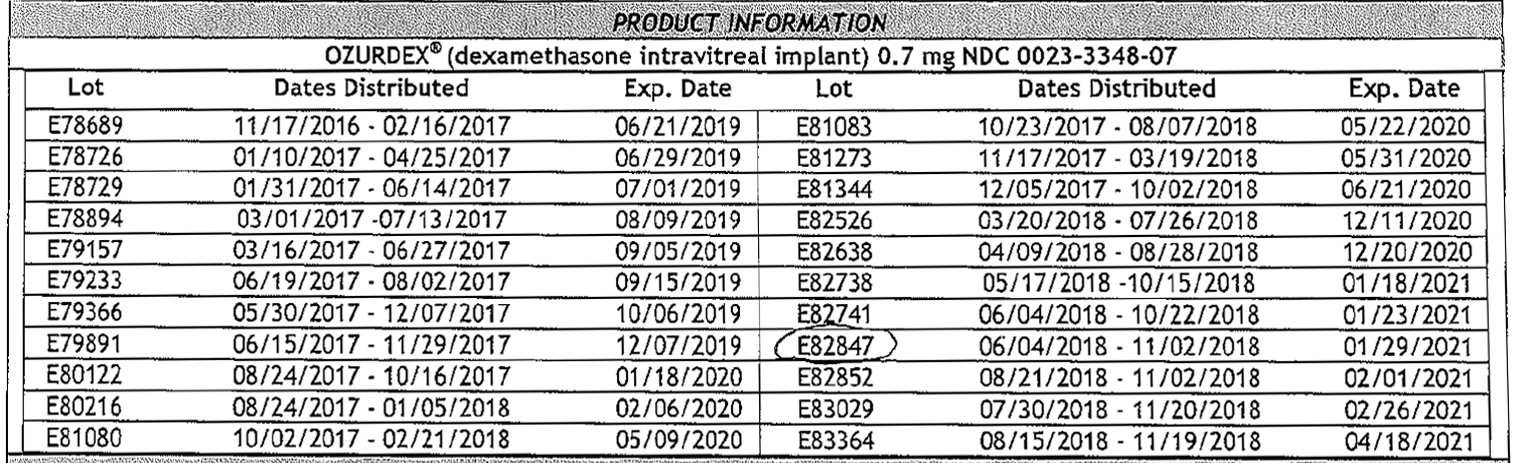
Allergan is recommending that health care providers stop using the recalled lots (shown below), and to return any affected batches. Physicians and patients can report adverse reactions or quality issues by 2 methods:
The Ophthalmic Mutual Insurance Company (OMIC) is offering risk management recommendations and a sample letter to patients on their website.