Introduction
Complications are more likely to occur with glaucoma surgery in children than in adults,1 largely due to anatomical factors related to ocular enlargement and the inherent characteristics of the pediatric eye. The thinned, stretched sclera of the buphthalmic eye, combined with the elasticity and low scleral rigidity of a pediatric eye, make these eyes prone to complications, especially hypotony. The potential for complications associated with glaucoma surgery in children cannot be underestimated. Significant intraoperative and postoperative complications in children are reported in the literature, especially with trabeculectomy2,3 and glaucoma drainage device (GDD) surgery,4,5 which makes modifications to these techniques mandatory. Hence, familiarity with buphthalmic eyes and a modified surgical technique performed meticulously are required to minimize complications. Also, the difficulty of examining children, especially in the postoperative period, makes it even more problematic to identify emerging complications in a timely fashion. Portable/hand held slit lamps tend to be the only way to get a better look at the anterior chamber, and these rarely give enough detail to quantify cell and flare assessment in such cases.
Considering the potential for complications with glaucoma surgery, it is paramount they be discussed with the child’s parents or caregivers as part of the process of consenting for surgery. Parents or caregivers must be alerted to signs that may suggest the onset of complications and of the possible need for a return to the operating suite if they occur. Furthermore, the potential for glaucoma surgery and its complications to affect the quality of life of children should not be overlooked.6
The best way to manage complications is to avoid them. This requires adequate assessment of the patient, including knowledge and review of past surgical history to prevent the repetition of a technique previously associated with a complication. It also demands preparation by the surgeon; problems must be anticipated and detailed consideration applied to avoid them. This section addresses the prevention and management of the more common and serious complications that may occur in children undergoing glaucoma surgery.
Infection
Ocular infection following glaucoma surgery can develop in a bleb (bleb-related infection) or can be associated with exposure of the tube or plate of a glaucoma drainage device (GDD). It is usually a late complication of surgery and is very serious because it can be rapidly blinding.
Bleb-related infection (BRI)
BRI refers to a spectrum of disease severity ranging from infection limited to the bleb to fulminant endophthalmitis. Blebitis is generally regarded as an isolated bleb infection without clinically apparent vitreous involvement, whereas bleb-related endophthalmitis (BRE) is generally regarded as extension of the infection into the eye (in which case a vitreous biopsy and intraocular antibiotics are indicated).7 BRI can occur following any filtration surgery in which there is a bleb such as trabeculectomy and combined trabeculotomy-trabeculectomy (CTT). Rarely, it may occur following trabeculotomy with inadvertent bleb formation.
Frequency
BRI is thought to occur more frequently in children, as compared to adults, due to children’s poor hygiene.8 However, rates vary depending on the study, from none to 17% in a study by Sidoti, in which there was a mean follow-up period of 28 months.9,10 Bleb-related endophthalmitis has been reported in up to 9% of pediatric mitomycin C (MMC) trabeculectomies.9,10
Pathogens
Studies from the adult literature report that BRI is caused by a different, more virulent spectrum of organisms (eg, streptococcal species, Haemophilus influenza, and Pseudomonas aeruginosa7,11,12) than the organisms that cause acute postsurgical endophthalmitis (usually gram positive organisms introduced at the time of surgery13). In the pediatric literature, the pathogens are often not reported. When they are reported, the organisms are consistent with those found in tidkadults.3
Risk factors
A number of risk factors for BRI exist, but the most common relate to bleb morphology: an avascular, thin-walled, cystic bleb that leads to compromised physical and immunological defenses against organisms (Figure 1). Other risk factors include chronic bleb leak,14 inferior placement of the trabeculectomy,15 contact lens use,11 16 and bacterial conjunctivitis.11 17,18 Children with filtering blebs, especially blebs that are “at-risk,” who experience bacterial conjunctivitis should be treated with a topical bactericidal antibiotic and closely observed during and after treatment for the development of intraocular inflammation. Furthermore, a history of prior bleb infection should prompt even greater vigilance, because prior infection has been calculated to increase the risk of developing endophthalmitis approximately 12 fold.17
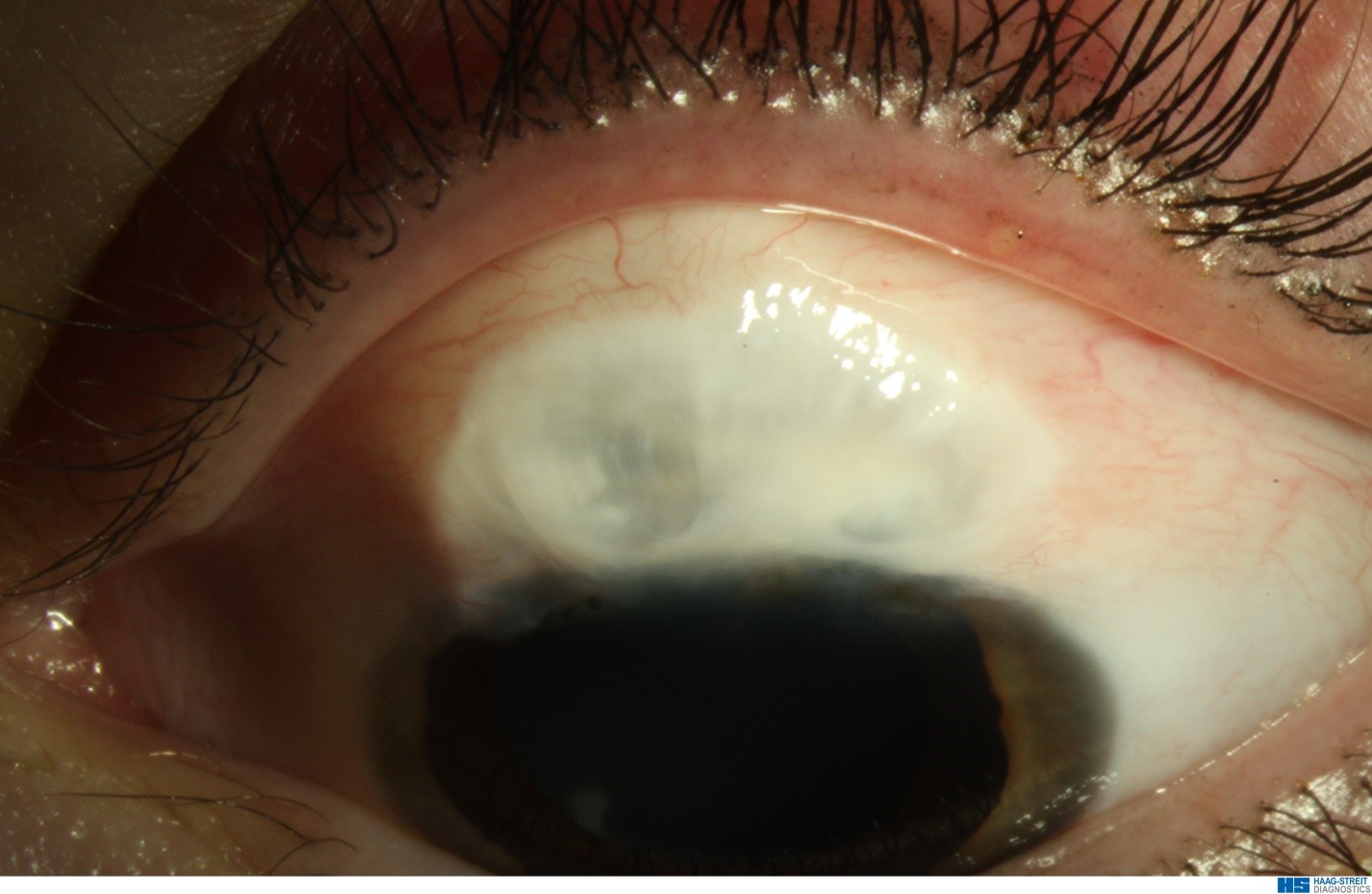
Figure 1. An at-risk bleb: avascular, cystic bleb at risk of infection. (Courtesy of Maria Papadopoulos, MD)
Clinical features
BRI is usually symptomatic and may present with foreign body sensation, photophobia, blurred vision, pain, conjunctival inflammation, or purulent discharge. A prodrome of a few days is characteristic of blebitis, whereas sudden onset and rapid progression suggests endophthalmitis. Children who present with a short prodrome of 24-48 hours should be closely observed for progression of signs. In early blebitis, there is intense conjunctival inflammation limited to the area immediately around the bleb, which helps distinguish it from generalized conjunctivitis, and this relative disparity alerts the clinician to the bleb as the source of the symptoms. The thin, cystic, avascular bleb will be white against the hyperemia of the surrounding conjunctiva, known as the “white on red” appearance (Figure 2). This can progress to a mucopurulent infiltrate of the bleb and a purulent discharge. A thin slit lamp beam through the bleb may show a hypopyon within it. There may be an associated bleb leak. Anterior chamber (AC) activity is variable and vitritis may be present, indicating endophthalmitis. A B-scan is indicated if the presence of vitritis cannot be clinically assessed. Expect to see low- to medium-density vitreal echoes with endophthalmitis. Conjunctival and eyelid cultures from BRE have been found to correlate poorly with intraocular cultures.11,12
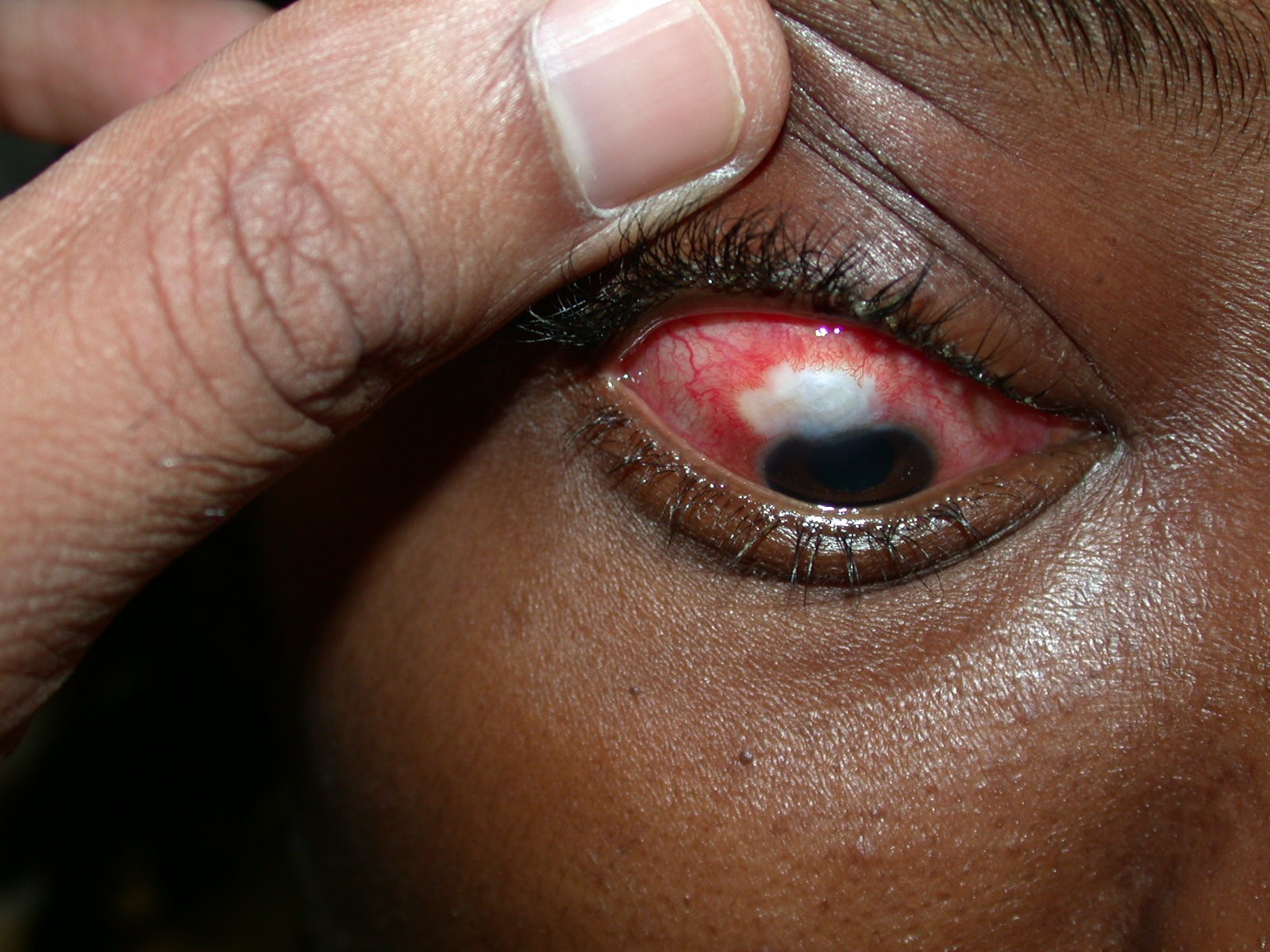
Figure 2. “White on red” bleb appearance associated with bleb-related infection. (Courtesy of Maria Papadopoulos, MD)
Management
Acute management is determined primarily by the stage of disease, by the severity of intraocular inflammation, and by the rapidity with which it is progressing. There are no randomized, controlled trials that have established the optimum antibiotic regimen for the treatment of BRI. However, topical and systemic broad-spectrum antibiotics are indicated to cover the diverse array of pathogens that may be responsible. The quinolones have a good broad-spectrum cover for gram-positive and gram-negative organisms. The fourth-generation quinolones (moxifloxacin, gatifloxacin, and besifloxacin) have better gram-positive coverage, including significant activity against Streptococcus pneumoniae and Staphylococcus species resistant to second- and third-generation quinolones.19 Later, the antibiotic regimen can be refined according to the patient’s response to treatment and to the culture and sensitivity results. Consider the addition of polymyxin B for multidrug-resistant bacteria and gram negative bacteria, and vancomycin for methicillin-resistant Staphylococcus aureus (MRSA).
Children with blebitis require frequent topical antibiotics (eg, moxifloxacin hourly day and night) and should be re-evaluated within 4 to 6 hours to check for signs of progression, such as increasing symptoms, deterioration of vision, and/or cellular activity in the aqueous or vitreous. Hospital admission should be considered for clinical or social reasons when there are concerns that adequate treatment cannot be administered at home. BRE must also be treated with intravitreal antibiotics once aqueous and vitreous samples have been taken. Topical steroids are also recommended once the course of the infection is clear.
Clear guidelines are lacking for the role of vitrectomy versus vitreous tap and intravitreal antibiotics alone in treatment of BRE. The results of the Early Vitrectomy Study20 cannot be generalized to all forms of endophthalmitis. Busbee21 and colleagues reported that patients with BRE who had prompt vitrectomy had better visual outcomes. In theory, vitrectomy decreases the bacterial load and associated toxins, helping to preserve retinal function. They suggested prompt vitrectomy, or vitrectomy in culture-positive patients who show no improvement after vitreous tap and injection. It is advisable to consider a vitrectomy if there is significant vitreous involvement. Furthermore, in children, vitrectomy may be the best way to obtain a satisfactory sample for culture and diagnostic purposes. The value of intravitreal steroids in the treatment of BRE has also not been established. Kangas and colleagues showed a trend toward a better visual outcome with intraocular steroid injection.22 The rationale is that the inflammatory response often causes more tissue destruction than the inciting infection.
Although blebs usually maintain filtration function after infection,10 a decision has to be made following an episode of BRI about whether surgical intervention is indicated to minimize the risk of further infection. Multiple factors must be considered, including whether a bleb extends into the interpalpebral fissure; whether there is poor contralateral vision, poor hygiene, or persistent bleb leak; whether the patient or caregivers are able to follow advice and act on the symptoms or signs of infection; and whether ophthalmic care is accessible. Usually this last factor, along with the young age of the patient, places them at lifelong further risk of infection and lowers the threshold for surgical intervention.
Bleb revision involving bleb excision and conjunctival advancement is the most definitive treatment.23 However, this alone will not prevent recurrence of a thin, avascular bleb if scleral thinning or a full-thickness sclerostomy is evident at the time of the revision and is not also addressed with a patch graft.
Prevention
The symptoms, signs, and significance of bleb-related infection must be made clear to the patient at risk of infection and to the parents or caregivers, who should be instructed to seek immediate attention.
Recent modifications to the pediatric trabeculectomy technique and to the intraoperative application of MMC have aimed to reduce the incidence of thin avascular blebs and their associated complications.24,25 Wells and colleagues, in a retrospective comparative study undertaken in children and young adults, demonstrated a significant reduction in the incidence of serious bleb-related complications with modifications such as a large area of anti-scarring treatment, a fornix-based conjunctival flap (ie, limbal incision hinged in the fornix) and fashioning a scleral flap to encourage posterior flow.26 Furthermore, releasable sutures should always be buried and antibiotics used when sutures are exposed. Anti-fibrotic agents should be cautiously and appropriately selected according to the patient’s risk factors for failure. Inferiorly placed blebs should be avoided, as should blebs with nasal and temporal extensions within the interpalpebral fissure.
The role of prophylactic antibiotics is controversial and has not been proven to decrease the incidence of infection. However, it may be appropriate in those children with “at-risk” blebs who may be unable to reach an ophthalmologist urgently for assessment and treatment (eg, while on vacation) to be given a broad-spectrum antibiotic to use if symptoms develop, until they can reach an ophthalmologist.
Prognosis
Long-term visual prognosis depends on the extent of the infection, the virulence of the organism, and the timing of therapy. BRI should be treated early and aggressively to maximize visual function.
Patients with culture-proven endophthalmitis, especially with Streptococci and gram-negative organisms such as Pseudomonas, generally have a very poor prognosis.2,11,12,21,27 This is in contrast to patients with blebitis, most of whom achieve vision back to or within one line of pre-infection visual acuity.9,12,17 Blebitis, being a precursor of endophthalmitis, is more effectively treated at an earlier stage, resulting in a better prognosis.7,17 Generally, visual acuity outcomes in BRE are worse than in acute onset endophthalmitis after cataract surgery.20
Endophthalmitis Associated With Glaucoma Drainage Devices (GDD)
Endophthalmitis associated with GDD is a rare but serious complication reported with all currently used GDDs.
Frequency
The exact incidence is not known but ranges from 0.8% to 6.3% with a mean of 2%.20 In a large series of 542 eyes with Ahmed implants, the rate of endophthalmitis for all ages over a 9-year follow-up period was found to be 1.7%; however, children had a five-times higher rate of endophthalmitis compared to adults (4.4% in children versus 0.9% in adults).28
Pathogens
The pathogens associated with GDD-related endophthalmitis tend to be similar to those associated with BRE, such as Pseudomonas aeruginosa, Haemophilus influenzae, and Streptococcus species.28,29
Risk factors
Endophthalmitis can occur early following initial GDD surgery30 or late from (i) surgery associated with tube repositioning,31 capsulectomy,32 and needling of the capsule33 and (ii) exposure of the device. (Figure 3) Endophthalmitis of a Baerveldt implant has also been reported, associated with a conjunctival buttonhole caused by a protruding suture securing the plate to the sclera.34
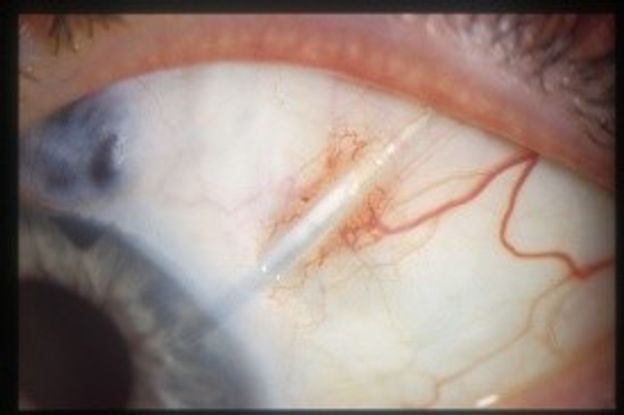
Figure 3. Exposed tube (Courtesy of Maria Papadopoulos, MD)
Although exposure can occur anywhere along the length of the tube or plate, it usually occurs over the tube at the limbus.29 Tube exposure is a major risk factor for endophthalmitis.28 Chen and colleagues reported 3 eyes with Ahmed tube exposure in a series of 52 pediatric eyes (5.8%), one of which developed endophthalmitis (1.9%).35 In a similar series of 60 eyes with Ahmed implants, the rate of tube exposure in children was higher at 12% and endophthalmitis at 3%.36 This emphasizes the importance of inspecting the overlying conjunctiva for tube exposure whenever the child is examined.
Clinical features
Clinical features include ocular pain, reduced vision, purulent discharge, anterior chamber activity, and possibly vitritis. If there is exposure of the implant, there may also be a leak and hypotony.
Management
Acutely, the management is the same as that for BRE with broad-spectrum topical, systemic, and, most importantly, intravitreal antibiotics once aqueous and vitreous samples have been taken.
It is unclear in the literature whether the implant should be removed. Removal of the implant in the acute phase is challenging because of excessive bleeding that occurs in an inflamed eye, but is also sometimes necessary if the infection does not resolve despite antibiotics. There are reports of successful treatment with the GDD in situ, but in most cases the implants are removed, as the GDD is seeded with the pathogen and the implant acts as a foreign body and is difficult to sterilize.29,36 Temporary removal of the tube from the AC, with subsequent reinsertion following successful treatment of endophthalmitis, is associated with recurrence of infection.31
Given the increased risk of endophthalmitis, if tube exposure is discovered, prompt surgical revision with a patch graft is necessary. Furthermore, the longer the conjunctiva remains open, the greater the opportunity for epithelial downgrowth at the site of exposure, which may cause recurrent exposure.37 Consideration at the time of surgery should be given to the cause of exposure. The tube may need to be re-sited more posteriorly from the limbus if the exposure is corneal. Fixing the tube to the sclera with 9-0 nylon, then placing a double layer of patch graft38 or using corneal tissue as a patch graft (which may be less susceptible to erosion), should be considered. Furthermore, avoidance of conjunctival incisions over the patch graft is advisable, as they are potential weak areas for further breakdown and exposure. Repair of the conjunctiva can be challenging, especially if there have been multiple previous operations. Conjunctival autografts, amniotic membrane grafts, or pedicle conjunctival flaps may be necessary in this situation.
Tube or plate extrusion (Figure 4) requires prompt removal. The tube should not be reinserted into the eye, because there is a risk of infection.
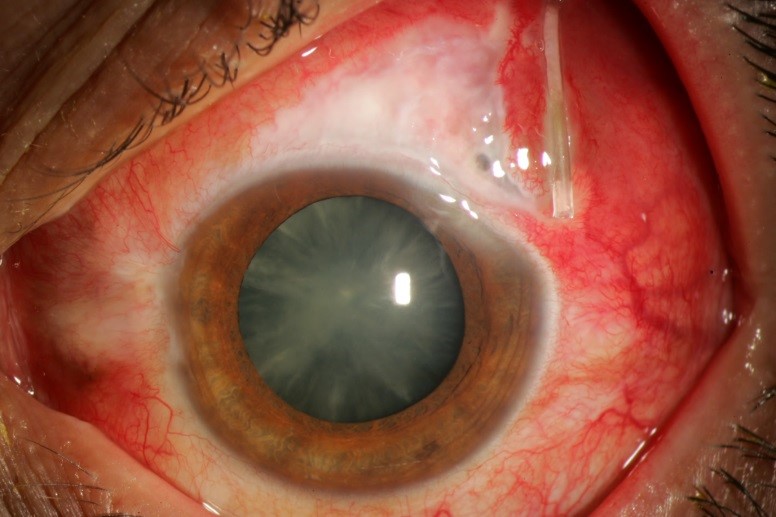
Figure 4. Extruded tube with infection – note purulent material within tube lumen. (Courtesy of Beth Edmunds, MD)
Prevention
As exposure is such a significant risk factor for GDD-related infection, measures to prevent or minimize this risk are very important.
The risk of tube exposure is reduced by avoiding a corneal tunnel that would not be covered by the patch graft and eyelid and therefore be more likely to erode. Furthermore, it is advisable to avoid a prominent bend in the tube at the limbus, which is more likely to become exposed from persistent rubbing of the eyelid. Constructing the tunnel at least 1-2 mm posterior to the limbus is advisable.
A patch graft over the tube is thought to significantly reduce the risk of tube exposure, although it can still occur despite the graft.39 Common patch materials include sclera, cornea, and pericardium.38 Thick amniotic membrane40 and dura mater41 have also been used. Suturing the patch well at the limbus to prevent posterior migration and tube exposure is important. Insertion of the tube through a long scleral tunnel is also thought to decrease the risk of tube exposure.42,43 In a retrospective study, with follow-up ranging from 6 months to four years, of 128 pediatric eyes in which Ahmed valves were placed using a long scleral tunnel technique without a patch graft, there were no cases of tube erosion.43 Attention to suturing technique can help minimize exposure. For example, securing the plate and tube well to the sclera may help reduce tube movement. Avoiding excessive conjunctival tension at the limbus may reduce the risk of early conjunctival retraction and subsequent tube exposure. Care should be given to rotating the plate-fixing suture ends through the eyelets of the plate to prevent the sutures from potentially causing a conjunctival buttonhole and access of pathogens into the eye.
Prognosis
The prognosis following endophthalmitis varies from maintaining pre-infection vision to phthisis and enucleation.29,32,36
Hypotony
There is a higher risk of hypotony in children due to their thin, elastic sclera and cornea, which gape when incised and tend to collapse at low pressures. Hypotony is a potentially major sight-threatening complication of surgery due to the risk of suprachoroidal hemorrhage in buphthalmic eyes that can occur intraoperatively or postoperatively. The risk is relatively low, but as with endophthalmitis, careful attention to technique is critical to avoid these devastating complications. In addition to hemorrhage, hypotony can result in a shallow or flat anterior chamber (AC). In phakic patients, this may precipitate or hasten cataract formation. If there is endothelial corneal touch, this can contribute to endothelial decompensation and ultimately to corneal failure. Children with aniridia are particularly vulnerable to this complication, as there is no intervening iris to help separate an anteriorly displaced lens and the cornea. Hypotony maculopathy and choroidal effusions can also develop. Children with Sturge-Weber syndrome who have choroidal hemangiomas are especially prone to large serous choroidal effusions, even at relatively normal intraocular pressures (IOPs), and to suprachoroidal hemorrhage in the early postoperative period. Uveitic patients also carry an increased risk of hypotony and its consequences, as ciliary body shutdown caused by surgically induced inflammation, or subsequent flare of uveitic disease, may also cause hypotony.
Frequency
Early hypotony following trabeculectomy is common and has been reported in almost 50% of cases.3 More recent literature suggests a lower incidence, around 10%.25 It can also be found after GDD surgery either due to overfiltration through the tube, or to leakiness around the tube in the scleral tunnel. Vigorous diode treatment, or diode in an eye with brittle aqueous production, may also result in hypotony. In the past, choroidal effusions (Figure 5) and flat anterior chambers associated with early hypotony following trabeculectomy surgery have been reported at a rate of 10%2 to 22%,10 respectively. With recent modifications to the trabeculectomy technique, these complications have been significantly reduced to a choroidal effusion rate of 10% and no cases of flat anterior chambers.25 Chronic hypotony associated with trabeculectomy can also occur and has been reported at a rate of 3-8%.9,44 Hypotony associated with a GDD ranges from 11% to 25%.45,46 Hypotony is rare after angle-based procedures, as these are designed as closed-globe surgeries, unless the wounds have not been securely sutured. Hypotony after transscleral cyclophotocoagulation with choroidal effusions has been reported in approximately 6% of cases.47
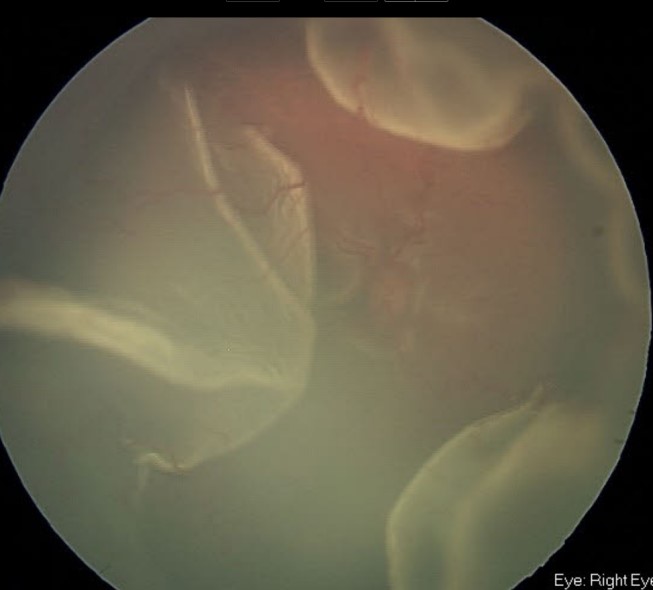
Figure 5. Florid serous choroidal effusions following filtering surgery in child with Sturge-Weber syndrome (Courtesy of Beth Edmunds, MD)
Risk factors
The anatomy and tissue characteristics of buphthalmic and pediatric eyes make them prone to hypotony. Uveitic eyes are also more likely to develop hypotony. Multiple diode treatments prior to insertion of a GDD may also predispose the eye to hypotony.
Management
Management of hypotony depends on the degree of hypotony and is targeted toward the cause of hypotony. It is best to manage the primary cause of hypotony first, as the resulting consequences of hypotony will resolve with treatment of the underlying cause. Buphthalmic eyes with significant hypotony should not be left to observation for very long, because there is a real risk of suprachoroidal hemorrhage.
Following trabeculectomy, early hypotony is often caused by overfiltration. If the IOP is low but there are no choroidal effusions, the patient can be managed conservatively with observation and reduced frequency of topical steroid use to encourage some healing. However, if the AC is shallow or flat and/or there are significant choroidal effusions, it is appropriate to consider injecting viscoelastic into the anterior chamber. It is also appropriate to reduce the frequency of topical steroid use, and to consider performing further surgery (such as the addition of further sutures to the scleral flap or the repair of a wound leak) to address the cause of hypotony.
With GDD surgery, early hypotony is usually due to leakage around the tube or failure of the flow restrictor in an Ahmed implant. Hypotony may occur with non–flow-restricted implants such as the Molteno or Baerveldt around the sixth postoperative week, when the external ligating suture dissolves. Usually the hypotony is short lived. If it persists, ligating sutures such as 9-0 prolene around the tube by an intracameral approach may be necessary.
Prevention
To prevent hypotony and suprachoroidal hemorrhage during surgery, the use of an AC maintainer is advised.
Postoperative hypotony following trabeculectomy can be minimized by fashioning as thick a scleral flap as possible with short radial cuts to create a valve effect and by minimizing leakage through the scleral flap sides: tight scleral flap lamellar sutures to avoid cheese-wiring, watertight conjunctival closure, and the use of a small amount of viscoelastic in the AC. Furthermore, using an appropriate concentration of MMC at the time of surgery is also important to minimize prolonged early hypotony.
A number of measures can be employed to reduce the risk of hypotony following GDD surgery. The leaflets of the Ahmed flow restrictor are designed to collapse together at lower IOPs, so this design offers the advantage of tubes without flow restriction (Baerveldt and Molteno). However, the flow restrictor can rarely fail, leading to hypotony. External ligation of the unrestricted implants is recommended to reduce aqueous flow in the early postoperative period and reduce this risk. An absorbable suture material (6-0 vicryl) may be used as an external ligature, which dissolves after 4-6 weeks to allow aqueous flow to return to the tube. In unrestricted implants, an intraluminal stent made of 3-0 supramid suture may be used in combination with or as an alternative to external ligation to reduce aqueous flow to avoid hypotony. Leaving some cohesive viscoelastic in the AC at the end of the case can help maintain the AC and the IOP in the first 24-48 hours after surgery. Despite these efforts, hypotony may still occur unless the scleral tunnel provides a snug watertight fit around the tube. Although the tube is 23G in size, a 25G needle is usually adequate for fashioning the tube entry site in children’s eyes, as the sclera is elastic. An alternative strategy to minimize hypotony takes advantage of the healing response by allowing the plate capsule to develop for a few weeks before allowing aqueous to flow. This is achieved with a two-staged procedure in which the GDD plate is positioned at the first surgery and the tubing inserted into the AC some weeks later at the second surgery.48
In uveitic patients or in infants with Sturge-Weber and choroidal hemangiomas, a small surface area GDD such as the Baerveldt 250 mm2 can be considered to minimize the risk of hypotony.
Aqueous Leaks
Aqueous leaks can occur at any time after surgery. Scleral and corneal elasticity make the pediatric eye vulnerable to early postoperative leaks if wounds have not been adequately sutured. It is important to recognize that straightforward incisions such as a paracentesis which, in adults, usually remains water-tight with stromal hydration, may not be sound in children if a similar technique is attempted. However, early postoperative limbal bleb leaks in fornix-based conjunctival flaps are uncommon, because children have a more pronounced healing response and more robust Tenon capsule than those in adults. Late bleb leaks may occur in avascular thin blebs. Exposure of the tube or plate of a GDD may also be associated with a leak.
The consequences of an aqueous leak can be profound, leading to hypotony and its associated sequelae, or infection. Preventative measures should be taken at the time of surgery, and leaks should be promptly addressed if discovered at any time postoperatively.
Frequency
Reported rates of late bleb leaks from the older pediatric trabeculectomy literature range from 3% to 23%.3,9,10 However, more recent studies advocating modifications to the pediatric trabeculectomy technique that minimize the occurrence of avascular thin blebs suggest lower rates of many of the traditional trabeculectomy complications, including leaks.26 In two recent studies using contemporary surgical techniques when performing MMC trabeculectomies in children, there were no early or late bleb leaks.25,49
Late leaks from GDD surgery are reported to range from none to approximately 4%.44,50
Risk factors
Factors predisposing to early aqueous leaks from wounds often relate to poor suturing technique. Excessive eye rubbing due to discomfort may lead to conjunctival wound dehiscence, which can be minimized by cutting suture ends short and having them covered well by a conjunctival frill when suturing a fornix-based conjunctival flap closed.
Bleb morphology is a major risk factor for the development of a late bleb leak in trabeculectomies. The “bleb at risk” is thin, avascular, and cystic, and is vulnerable to minor trauma and infection, which can lead to tissue breakdown. Leaks may therefore both predispose to and result from BRI, and may be intermittent.
With GDDs, the main risk factors leading to leaks are tube exposure or erosion (see the section titled Endophthalmitis Associated With Glaucoma Drainage Devices [GDD], above).
Management
An early postoperative leak from an inadequately closed corneal or conjunctival incision may be easily remedied by resuturing, with modifications if needed, as described below in the section on prevention.
Chronic late leaks are usually refractory to conservative measures such as a bandage contact lens due to poor tissue integrity and established misdirection of aqueous flow through the leak. Often surgical revision is necessary, with excision of the unhealthy conjunctival tissue so that the fresh edges of the new wound are well vascularized and thick. To allow conjunctival closure without excessive tension, extensive undermining of conjunctiva and Tenon capsule beyond the area of the cystic bleb and occasionally separation of Tenon capsule from the conjunctiva may be required. Partial-thickness conjunctival incisions deep in the fornix are also sometimes necessary to release enough tissue for closure. Consideration should also be given to addressing any scleral defects with a patch graft to prevent the recurrence of a thin, avascular, cystic bleb in trabeculectomies, or in the case of a GDD, changing the tube to a position less likely to erode.
The repair of a persistent bleb leak associated with infection should be delayed until the infection has completely resolved, which may be several weeks or months after the acute episode.
Management of leaks associated with GDD exposure is discussed above.
Prevention
The mainstay of management of aqueous leaks is prevention. The opposing edges of incisions in buphthalmic eyes with thin sclerae and corneas do not naturally seal in children as they do in adults. Therefore, all paracenteses should be sutured closed and, if the seal is in doubt, more than one suture should be placed. The tension on these sutures should also be greater than that in adults, as the sutures loosen after a few days in children. Locking suture techniques may therefore be useful. Allowing the eye to soften a little at the time of suturing makes it possible to tighten sutures without cheese-wiring though thin tissue. Passing the needle at partial thickness rather than full thickness through the tissue can also avoid aqueous seepage around the needle track. In very thin gaping or friable tissues where the needle track itself creates leaks, it is sometimes useful to use a round-bodied rather than a cutting needle, though these are harder to pass through the tissue, and the knots cannot be turned and so should be trimmed flush. Using an AC maintainer with balanced salt solution (BSS) to keep the eye inflated during surgery (rather than viscoelastic) also allows assessment of a wound’s integrity, as BSS leakage from suture tracks or subtle wound leaks will be detected. If still in doubt, using a fluorescein strip and illuminating with the cobalt blue filter from the operating microscope can also help determine the integrity of wound closure. Using a sealant may provide additional protection but has not been studied in children with glaucoma and should not be relied upon as a primary closure technique. The same applies to conjunctival wound closure. Meticulous suturing of a fornix-based conjunctival flap at the limbus is required after filtering surgeries.
Avoidance of avascular, cystic blebs associated with bleb leaks and infection is achieved by treating a large area with anti-fibrotic agents (2-3 clock hours), and by fashioning the scleral flap with short radial cuts that do not extend the full length of the scleral flap to the limbus in order to encourage posterior flow; this is discussed in the trabeculectomy section.
Minimizing GDD exposure is discussed above.
Prognosis
The prognosis of a leak will depend on whether it in itself leads to further complications such as hypotony and infection, both of which are addressed elsewhere in this section. Unfortunately, repairing the leak may lead to loss of IOP control, so one of the consequences of leaks ultimately can be uncontrolled glaucoma and its sequelae.
Corneal Decompensation
Failure of endothelial cell function leads to corneal decompensation and edema in both the native cornea and the transplanted cornea. Children with glaucoma may have compromised corneal function as part of the glaucomatous process itself or as a complication of glaucoma surgery.
Frequency
Corneal decompensation is one of the main late complications of GDD in children. Tube-corneal touch, a causative factor, is more common in buphthalmic eyes and has been reported as 20% in some series.45 Infants especially may be at higher risk, with one paper reporting that tube surgery in children less than two years of age required tube repositioning in 35% of the cohort because of near or actual tube-corneal touch.44 The corneal changes can vary from mild localized edema over the tube to extensive corneal decompensation or corneal graft failure.
Risk factors and mechanisms
Corneal decompensation can occur early following intraoperative trauma by a surgical instrument such as a Descemet tear from a trabeculotome or a goniotomy blade. The extent of the trauma will have varying effects in the short term on endothelial function with overlying stromal edema, which may or may not resolve.
Late postoperative corneal decompensation can result following GDD surgery where the tube is positioned in close proximity to the endothelium at the time of initial surgery, or if malposition develops later. The stretched limbal anatomy can relax a little once IOP is brought under control, and a tube that may have appeared well positioned away from the cornea at surgery can end up being closer to the endothelium than anticipated. With time and tissue remodeling, the tube may assume its original straight habitus, which can result in anterior vaulting of the tube tip, bringing it closer to the endothelium (Figure 6). Blinking movements, eye rubbing, and eye squeezing can contribute to intermittent endothelial trauma from a GDD tube tip.
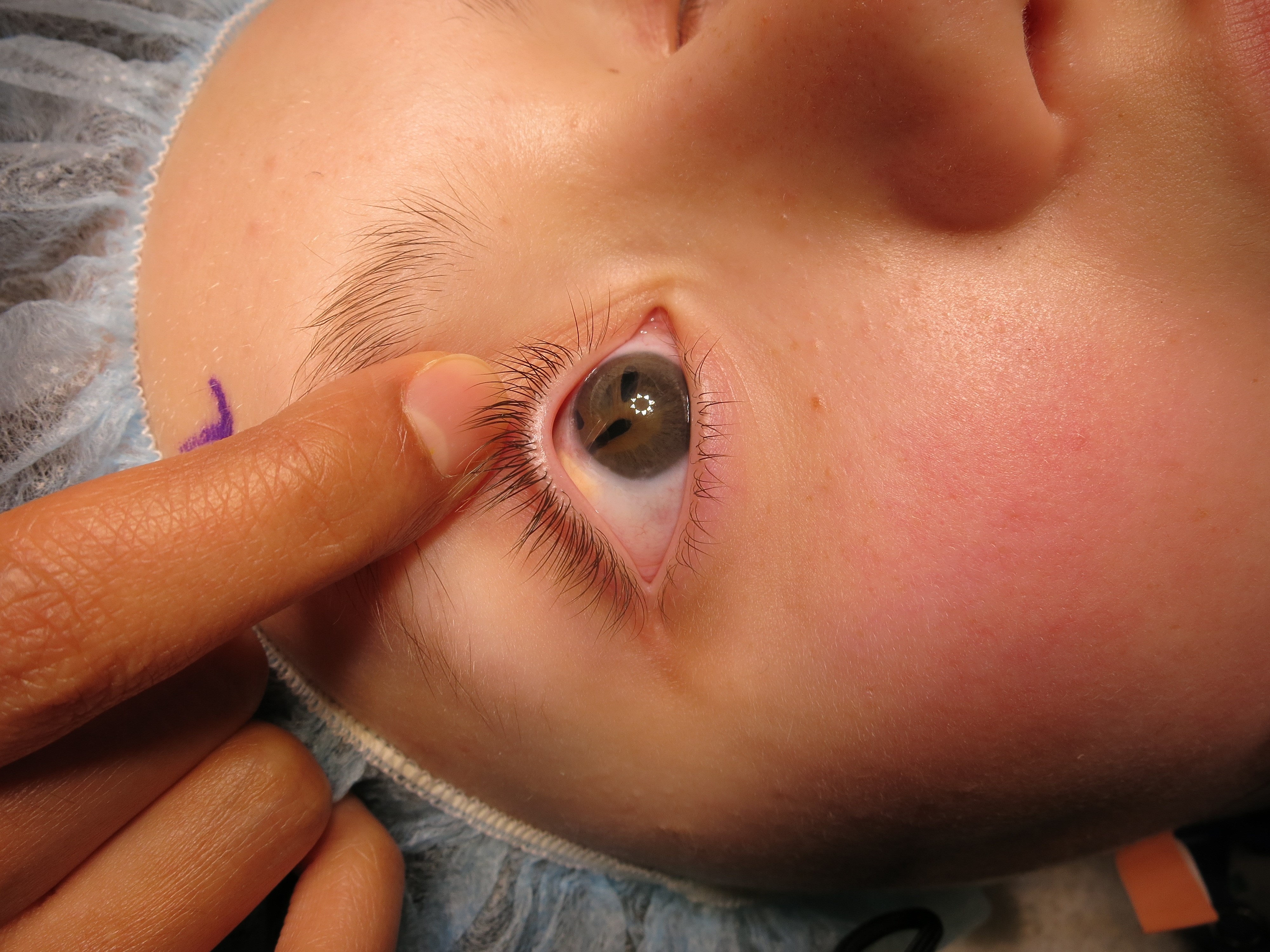
Figure 6. Anterior vaulting of tube tip putting endothelium at risk of tube-corneal touch. (Courtesy of Beth Edmunds, MD)
Management
Collaboration with a corneal specialist is important in managing corneal disease following glaucoma surgery. In young children, corneal decompensation brings with it the added risk of amblyopia. With early decompensation, trimming or moving a tube may help slow the progression of corneal failure. Keeping IOP as low as possible will assist endothelial cell function. With established corneal decompensation, the threshold at which to perform corneal surgery will depend on many factors. At the time of corneal surgery, it is important to consider addressing the tube by trimming the tube tip or repositioning it. Corneal surgery may also be considered to reduce discomfort associated with corneal decompensation and its impact on the child’s quality of life. In the early stages of decompensation, lubricants or NaCl 5%-10% may be helpful. When decompensation is advanced, surgery must be considered: either Descemet stripping automated endothelial keratoplasty (DSAEK) or penetrating keratoplasty.
Prevention
As many of the risk factors for anterior tube migration, corneal tube touch, and associated corneal decompensation are similar, steps to avoid or minimize these complications are discussed together in the section on prevention of tube migration, below.
Tube Migration
Tubes may lengthen in the AC because of migration of the plate anteriorly toward the limbus if it is not properly secured to the sclera, or with lowering of the IOP. Tubes may also retract with uncontrolled IOP due to an increase in the size of the eye, giving the impression of “shortening” in the AC, and they may ultimately retract from the AC altogether and cease to function. Tube-corneal touch refers to either the tip of the tube or its length touching the endothelium.
Frequency
Tube migration is one of the more common complications of tube surgery and can be seen with any of the currently available GDDs. There are reports of migration occurring in up to 35%,51 and corneal touch in up to 20% of Ahmed implants.48,50,52,53
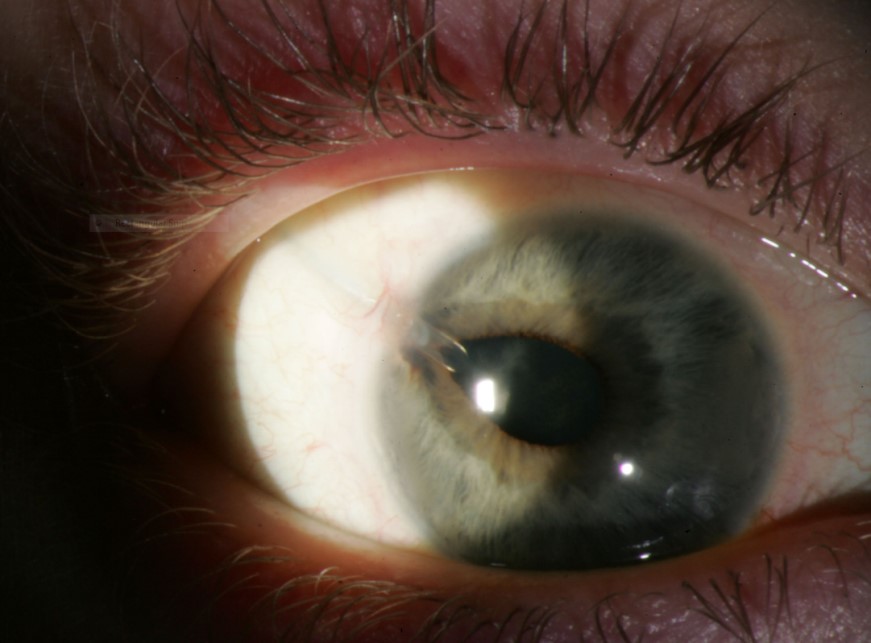
Figure 7. Dyscoria from tube. (Courtesy of Beth Edmunds, MD)
Risk factors
Tube migration and corneal touch are more likely in buphthalmic eyes. As there is some reversibility of pediatric scleral stretching with IOP lowering, the early postoperative position of the tube may be more anterior than was apparent during surgery when the tissues were more on stretch. The low scleral rigidity of the buphthalmic eye, memory in the tube material, and continued globe growth may contribute to this ongoing process of anterior migration.45
Vigorous eye rubbing has been suggested as a contributory mechanism for anterior migration of tubes.45
Management
Anterior tube migration will need tube revision (trimming or moving tube insertion) if it is associated with tube-corneal touch and corneal decompensation. Posterior tube migration with retraction from the AC is often associated with loss of IOP control, making revision with a tube extender,54 intravenous catheter (22 gauge),55 or a second tube necessary.
Prevention
Many of the maneuvers to prevent tube migration are the same as those for prevention of tube-related corneal decompensation, and so are addressed together in this section. The tube entrance to the AC should be placed as far posterior to the limbus as possible and covered by a patch graft. A long scleral tunnel may help secure it. The tunnel should be fashioned to direct the tube parallel to the iris once it is in the AC. The plate should be securely sutured to sclera, and sutures of 9-0 nylon placed along the tube length to help anchor it to the sclera. A patch graft will also help secure it, as well as cover the extraocular length of the tube. Placing the Baerveldt plate behind the muscles to prevent anterior migration is preferable.
It is important to ensure that patients and caregivers know to warn children to avoid pressing on the globe and rubbing their eyes, though these instructions are understandably difficult to enforce. Good surgical technique with well-buried sutures, limbal-shelved patch graft material, smooth limbal edges, and well-covered tube hardware will contribute to a comfortable eye that will be less likely to provoke rubbing.
Regular postoperative checks of tube position and behavior are important to detect anterior migration, corneal touch, or early corneal decompensation. Watching the tube’s stability in the AC while applying gentle digital pressure to the globe through the upper lid can give a sense of whether it maintains a distance from the endothelium, or rocks forwards towards the endothelium. Subtle disturbance of the endothelium or overlying stromal opacification in an area corresponding to the anterior extent of the rocking tube tip indicate corneal tube touch and tube repositioning or trimming should be performed.
References
- Chen TC, Chen PP, Francis BA, et al. Pediatric glaucoma surgery: a report by the American Academy of Ophthalmology. 2014;121:2107-2115.
- Susanna R Jr, Oltrogge EW, Carani JC, Nicolela MT. Mitomycin as adjunct chemotherapy with trabeculectomy in congenital and developmental glaucomas. J Glaucoma. 1995;4:151-157.
- Freedman SF, McCormick K, Cox TA. Mitomycin C-augumented trabeculectomy with postoperative wound modulation in pediatric glaucoma. J AAPOS. 1999;3:117-124.
- O'Malley Schotthoefer E, Yanovitch TL, Freedman SF. Aqueous drainage device surgery in refractory pediatric glaucoma: II. Ocular motility consequences. J AAPOS. 2008;12(1):40-45.
- Ou Y, Yu F, Law SK, Coleman AL, Caprioli J. Outcomes of Ahmed glaucoma valve implantation in children with primary congenital glaucoma. Arch Ophthalmol. 2009;127:1436-1441.
- Zhang XL, Du SL, Ge J, et al. [Quality of life in patients with primary congenital glaucoma following antiglaucoma surgical management]. Zhonghua Yan Ke Za Zhi. 2009;45:514-521.
- Brown RH, Yang LH, Walker SD, Lynch MG, Martinez LA, Wilson LA. Treatment of bleb infection after glaucoma surgery. Arch Ophthalmol. 1994;112(1):57-61.
- Golde KT, Gardiner MF. Bacterial conjunctivitis in children: a current review of pathogens and treatment. Int Ophthalmol Clin. 2011;51(4):85-92.
- Sidoti PA, Belmonte SJ, Liebmann JM, Ritch R. Trabeculectomy with mitomycin-C in the treatment of pediatric glaucomas. 2000;107:422-429.
- Beck AD, Wilson WR, Lynch MG, Lynn MJ, Noe R. Trabeculectomy with adjunctive mitomycin C in pediatric glaucoma. Am J Ophthalmol. 1998;126(5):648-657.
- Mandelbaum S, Forster RK, Gelender H, Culbertson W. Late onset endophthalmitis associated with filtering blebs. 1985;92(7):964-972.
- Ciulla TA, Beck AD, Topping TM, Baker AS. Blebitis, early endophthalmitis, and late endophthalmitis after glaucoma-filtering surgery. 1997;104(6):986-995.
- Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122(1):1-17.
- Soltau JB, Rothman RF, Budenz DL, et al. Risk factors for glaucoma filtering bleb infections. Arch Ophthalmol. 2000;118(3):338-342.
- Caronia RM, Liebmann JM, Friedman R, Cohen H, Ritch R. Trabeculectomy at the inferior limbus. Arch Ophthalmol. 1996;114(4):387-391.
- Bellows AR, McCulley JP. Endophthalmitis in aphakic patients with unplanned filtering blebs wearing contact lenses. 1981;88(8):839-843.
- Waheed S, Liebmann JM, Greenfield DS, et al. Recurrent bleb infections. Br J Ophthalmol. 1998;82(8):926-929.
- Katz LJ, Cantor LB, Spaeth GL. Complications of surgery in glaucoma. Early and late bacterial endophthalmitis following glaucoma filtering surgery. 1985;92(7):959-963.
- Mather R, Karenchak LM, Romanowski EG, Kowalski RP. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol. 2002;133(4):463-466.
- Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113(12):1479-1496.
- Busbee BG, Recchia FM, Kaiser R, Nagra P, Rosenblatt B, Pearlman RB. Bleb-associated endophthalmitis: clinical characteristics and visual outcomes. 2004;111(8):1495-1503.
- Kangas TA, Greenfield DS, Flynn HW, Jr, Parrish RK, 2nd., Palmberg P. Delayed-onset endophthalmitis associated with conjunctival filtering blebs. 1997;104(5):746-752.
- Burnstein AL, WuDunn D, Knotts S, Catoira Y, Cantor L. Conjunctival advancement versus nonincisional treatment for late-onset glaucoma filtering bleb leaks. 2002;109(1):71 - 75
- Khaw PT, Chiang M, Shah P, Sii F, Lockwood A, Khalili A. Enhanced Trabeculectomy: The Moorfields Safer Surgery System. Dev Ophthalmol. 2012; 50:1-28.
- Jayaram H, Scawn R, Pooley F, et al. Long-Term Outcomes of Trabeculectomy Augmented with Mitomycin C Undertaken within the First 2 Years of Life. 2015;122:2216-2222.
- Wells AP, Cordeiro MF, Bunce C, Khaw PT. Cystic bleb formation and related complications in limbus - versus fornix-based conjunctival flaps in pediatric and young adult trabeculectomy with mitomycin C. 2003;110(11):2192-2197.
- Song A, Scott IU, Flynn HWJ, Budenz DL. Delayed-onset bleb-associated endophthalmitis: clinical features and visual acuity outcomes. 2002;109(5):985-991.
- Al-Torbak AA, Al-Shahwan S, Al-Jadaan I, Al-Hommadi A, Edward DP. Endophthalmitis associated with the Ahmed glaucoma valve implant. British J Ophthalmol. 2005;89(4):454-458.
- Gedde SJ, Scott IU, Tabandeh H, et al. Late endophthalmitis associated with glaucoma drainage implants. 2001;108(7):1323-1327.
- Nguyen QH, Budenz DL, Parrish RK, 2nd. Complications of Baerveldt glaucoma drainage implants. Arch Ophthalmol. 1998;116(5):571-575.
- Fanous MM, Cohn RA. Propionibacterium endophthalmitis following Molteno tube repositioning. J Glaucoma. 1997;6(4):201-202.
- Djodeyre MR, Peralta CJ, Abelairas GJ. Clinical evaluation and risk factors of time to failure of Ahmed glaucoma valve implant in pediatric patients. 2001;108(3):614-620.
- Chen PP, Palmberg PF. Needling revision of glaucoma drainage device filtering blebs. 1997;104(6):1004-1010.
- Francis BA, DiLoreto DA Jr, Chong LP, Rao N. Late-onset bacteria endophthalmitis following glaucoma drainage implantation. Ophthalmic Surg Lasers Imaging. 2003;34(2):128-130.
- Chen TC, Bhatia LS, Walton DS. Ahmed valve surgery for refractory pediatric glaucoma: a report of 52 eyes. J Pediatr Ophthalmol Strabismus. 2005;42(5):304-305.
- Morad Y, Donaldson CE, Kim YM, Abdolell M, Levin AV. The Ahmed drainage implant in the treatment of pediatric glaucoma. Am J Ophthalmol. 2003;135(6):821-829.
- Sidoti PA, Minckler DS, Baerveldt G, Lee PP, Heuer DK. Epithelial ingrowth and glaucoma drainage implants. 1994;101(5):872-875.
- Lankaranian D, Reis R, Henderer JD, Choe S, Moster MR. Comparison of single thickness and double thickness processed pericardium patch graft in glaucoma drainage device surgery: a single surgeon comparison of outcome. J Glaucoma. 2008;17(1):48-51.
- Fechter HP, Parrish RK 2nd. Preventing and treating complications of Baerveldt glaucoma drainage device surgery. Int Ophthalmol Clin. 2004;44(2):107-136.
- Anand A, Sheha H, Teng CC, J.M. L, Ritch R, Tello C. Use of amniotic membrane graft in glaucoma shunt surgery. Ophthalmic Surg Lasers Imaging. 2011;42(3):184-189.
- Zalta AH. Long-term experience of patch graft failure after Ahmed Glaucoma Valve® surgery using donor dura and sclera allografts. Ophthalmic Surg Lasers Imaging. 2012;43(5):408-415.
- Ollila M, Falck A, Airaksinen PJ. Placing the Molteno implant in a long scleral tunnel to prevent postoperative tube exposure. Acta Ophthalmol Scand. 2005;83:302–305.
- Albis-Donado O, Gil-Carrasco F, Romero-Quijada R, Thomas R. Evaluation of Ahmed glaucoma valve implantation through a needle-generated scleral tunnel in Mexican children with glaucoma. Indian J Ophthalmol. 2010;58(5):365-373.
- Beck AD, Freedman S, Kammer J, Jin J. Aqueous shunt devices compared with trabeculectomy with Mitomycin-C for children in the first two years of life. Am J Ophthalmol. 2003;136(6):994-1000.
- Nassiri N, Nouri-Mahdavi K, Coleman AL. Ahmed glaucoma valve in children: A review. Saudi J Ophthalmol. 2011;25(4):317-327.
- Rolim de Moura C, Fraser-Bell S, Stout A, Labree L, Nilfours M, Varma R. Experience with the Baerveldt glaucoma implant in the management of pediatric glaucoma. Am J Ophthalmol. 2005;139(5):847-854.
- Autrata R, Rehurek J. Long-term results of transscleral cyclophotocoagulation in refractory pediatric glaucoma patients. 2003;217:393-400.
- Hill RA, Heuer DK, Baerveldt G, Minckler DS, Martone JF. Molteno implantation for glaucoma in young patients. 1991;98:1042-1046.
- Low S, Hamada S, Nischal KK. Antimetabolite and releasable suture augmented filtration surgery in refractory pediatric glaucomas. J AAPOS. 2008;12(2):166-172.
- Englert JA, Freedman SF, Cox TA. The Ahmed valve in refractory pediatric glaucoma. Am J Ophthalmol. 1999;127(1):34-42.
- Al-Mobarak F, Khan AO. Two-year survival of Ahmed valve implantation in the first 2 years of life with and without intraoperative mitomycin-C. 2009;116:1862-1865.
- Mullaney PB, Selleck C, Al-Awad A, Al-Mesfer S, Zwaan J. Combined trabeculotomy and trabeculectomy as an initial procedure in uncomplicated congenital glaucoma. Arch Ophthalmol. 1999;117(4):457-560.
- Fellenbaum PS, Sidoti PA, Heuer DK, Minckler DS, Baerveldt G, Lee PP. Experience with the Baerveldt implant in young patients with complicated glaucomas. J Glaucoma. 1995;4:91-97.
- Sarkisian SR, Netland PA. Tube extender for revision of glaucoma drainage implants. J Glaucoma. 2007;16(7):637-639.
- Bansal A, Fenerty CH. Extension of retracted glaucoma drainage tube using a 22-gauge intravenous catheter in complex pediatric glaucoma. J Glaucoma. 2010;19(4):248-251.