The links to each individual chapter of the Color Atlas of Gonioscopy are available at the Chapters link, below.
Primary Infantile Glaucoma
Primary infantile glaucoma results from failure of the angle to develop normally. The outflow of aqueous humor through the incompletely developed trabecular meshwork is impaired, resulting in increased intraocular pressure. Unlike the adult eye, the infant eye stretches in response to this elevated pressure. The infant cornea also develops edema at relatively lower pressures than the adult cornea.
The diagnosis of infantile glaucoma is usually made when parents notice photophobia with blepharospasm, tearing, cloudy corneas, or large eyes. Although the disease can present at any time within the first three years of life, symptoms usually develop by the age of three months and the diagnosis is usually made by the end of the child’s first year. Most cases of primary infantile glaucoma are bilateral. It is inherited in an autosomal recessive pattern, and a percentage of cases are associated with mutations in the CYP1B1 gene (Stoilov et al, 1997).
On physical examination, the eyes are often observed to be large (buphthalmos), with large corneas (7‑1). The corneas are edematous (7‑2) and may show breaks in Descemet’s membrane (Haab’s striae), which appear as parallel lines with a horizontal or circumferential orientation (7‑3). Gonioscopic examination requires sedation or general anesthesia. The examination is usually performed with a direct goniolens, such as a Koeppe, Hoskins-Barkan, or Swan-Jacobs lens (see 3‑5 to 3‑15). Gonioscopy is made difficult by the lightly pigmented, amorphous appearance of infant angles and by the corneal edema that is often present in infantile glaucoma.

7-1 Nine-month-old girl with bilateral primary infantile glaucoma. Her horizontal corneal diameters are markedly enlarged at 16 mm. Note the stromal scarring of the corneas.
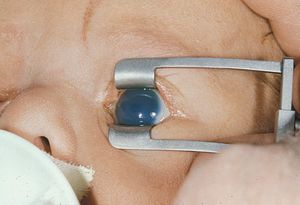
7-2 Infant with newly diagnosed primary infantile glaucoma demonstrating corneal edema.
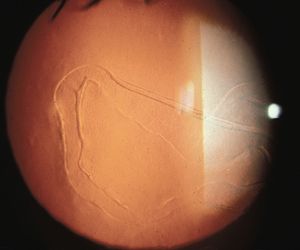
7-3 Haab’s striae in primary infantile glaucoma. These breaks in Descemet’s membrane are usually oriented horizontally (as seen here) or circumferentially. Vertical breaks may be seen in obstetrical injuries following forceps deliveries.
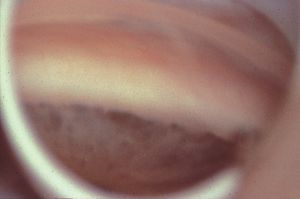
7-4 Angle of infant with primary infantile glaucoma viewed through a Swan-Jacobs lens. Note the high and irregular insertion of the iris, which covers the scleral spur. There are thin, pigmented areas in the peripheral iris. The trabecular meshwork is nonpigmented. (Courtesy of Randall E. Verdick.)
The angles in primary infantile glaucoma are immature (7‑4 to 7‑6). They demonstrate a rather flat iris configuration. The periphery of the iris is thin and the deeper pigment epithelium may show through in areas. There is a high, often irregular, insertion of the iris into the angles. The trabecular meshwork has a generalized sheen due to the thick trabecular beams. This gives the appearance of a membrane, which is referred to as Barkan’s membrane—although there is no histologic evidence for such a membrane (Anderson, 1981). The radial blood vessels of the iris are often visible and the major arterial circle can often be seen (7‑7) (Worst, 1966).
Viewed histopathologically (7‑8), the trabecular pillars are thick. The anterior ciliary body and iris insert over the posterior trabecular meshwork. There is histopathological and clinical evidence of traction on the pillars. The traction may cause compaction of the trabecular spaces, with impairment of aqueous outflow. Direct gonioscopy plays a key role in the surgical treatment of primary congenital glaucoma. Both goniotomy and trabeculotomy achieve their common goal of lowering resistance to aqueous outflow by cutting through the tight pillars and relieving the compaction of the trabecular spaces (Anderson, 1981). Goniotomy involves using a small blade or needle to cut through the trabecular meshwork. This requires a clear view of the angle via direct gonioscopy, which is sometimes not possible due the associated corneal edema. In cases where the corneal edema precludes adequate viewing of the angle, trabeculotomy is the preferred procedure. Trabeculotomy requires canalization of Schlemm’s canal externally, which can be extremely challenging.
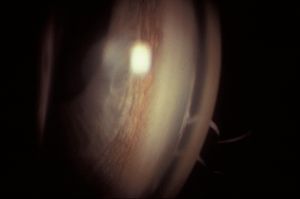
7-5 Temporal angle of 32-year-old with primary infantile glaucoma. There is a high, scalloped iris insertion and lightly pigmented trabecular meshwork.
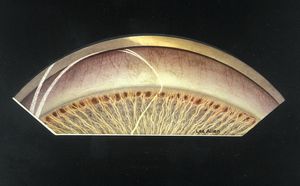
7-6 Fifteen-year-old with primary infantile glaucoma. She was first seen at age 6 with severe buphthalmos. There is generalized atrophy of the iris with islands of visible pigment epithelium. The iris inserts anterior to the scleral spur. The cornea anterior to the trabecular meshwork is opaque and thin.

7-7 The angle of a young adult with primary infantile glaucoma. An undulating greater circle of the iris is visible in the angle.
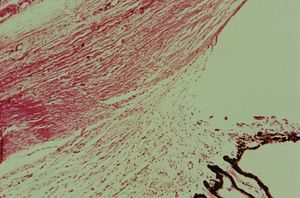
7-8 Histopathology of primary infantile glaucoma. The iris and anterior ciliary body cover the scleral spur and posterior trabecular meshwork. The intratrabecular spaces are compacted. (Courtesy of the National Museum of Health and Medicine, Armed Forces Institute of Pathology.)
Posterior Embryotoxon
Posterior embryotoxon is the name given to a prominent and anterior Schwalbe’s line. This can be seen on slit-lamp examination and is found in about 10% of the general population (7‑9). On gonioscopy, Schwalbe’s line is observed to be prominent and to protrude into the anterior chamber as a ring (7‑10). The ring of tissue may be partial or may extend through 360°. Although an extreme variation of it is present in most cases of the Axenfeld-Rieger syndrome, it is usually an isolated finding. Isolated posterior embryotoxon is not associated with an increased risk of glaucoma.
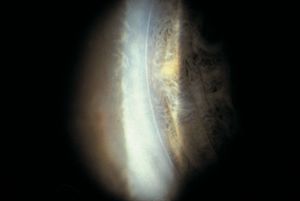
7-9 Posterior embryotoxon. An anterior and prominent Schwalbe’s line is a frequent finding in slit-lamp examination of normal individuals.
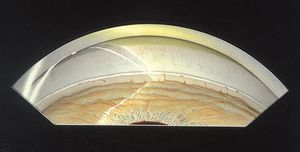
7-10 Posterior embryotoxon seen gonioscopically as a prominent Schwalbe’s line. There are fine iris processes in this eye.
Axenfeld-Rieger Syndrome
The family of disorders known as the Axenfeld-Rieger syndrome includes Axenfeld anomaly, Rieger anomaly, and Rieger syndrome. These are developmental abnormalities that represent a spectrum of anatomic changes ranging from localized ocular abnormalities to systemic abnormalities. Like primary infantile glaucoma, they are developmental disorders of the anterior chamber, but they are morphologically distinct from primary infantile glaucoma. These conditions are usually bilateral and are inherited in an autosomal dominant manner. Mutations in the genes PITX2 and FOXC1 (formerly FKHL7) have both been shown to cause Axenfeld- Rieger syndrome and other similar conditions (Alward et al, 1998, and Nishimura et al, 1998). Glaucoma develops in about 50% of cases, generally in late childhood or early adulthood (Shields et al, 1985).
Axenfeld anomaly is characterized by posterior embryotoxon with multiple iris processes that extend to Schwalbe’s line (7‑11 and 7‑12). This line may be dramatically thickened (7‑13). It may be suspended from the cornea by a fine membrane and can break free from the cornea, leaving portions hanging into the anterior chamber (7‑14) (Wolter et al, 1967). The iris processes can be broad (7‑15) or lacy and delicate. The changes to the angle generally affect the entire angle, but they may also be restricted to a small area (7‑16). The iris usually inserts anteriorly and obscures the scleral spur.
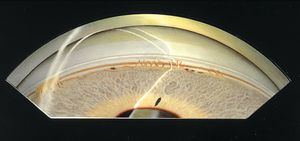
7-11 Axenfeld anomaly. Multiple iris processes have formed between the iris and a prominent Schwalbe’s line. There is a broad area of iris adhesion to Schwalbe’s line on the left of the illustration. (Reprinted with permission. Arch Ophthalmol. 1955;53:767–782. Copyright © June 1955, American Medical Association. All Rights Reserved.)
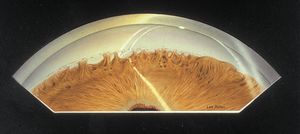
7-12 Axenfeld anomaly with dense iris adhesions that almost completely cover the trabecular meshwork. Particles of pigment are deposited along a very prominent Schwalbe’s ring. (Reprinted with permission. Arch Ophthalmol. 1955;53:767–782. Copyright © June 1955, American Medical Association. All Rights Reserved.)
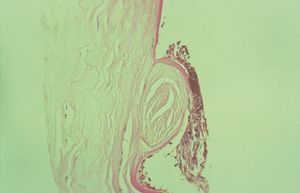
7-13 Histopathology of Axenfeld-Rieger anomaly. Note the markedly thickened Schwalbe’s line (S) with adherent iris process (arrow).
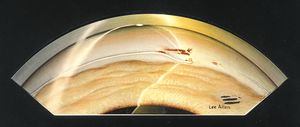
7-14 Axenfeld anomaly with partially detached Schwalbe’s ring within the anterior chamber. (Reprinted with permission. Arch Ophthalmol. 1955;53:767–782. Copyright © June 1955, American Medical Association. All Rights Reserved.)

7-15 Broad iris process and evident posterior embryotoxon in a patient with a known FOXC1 mutation.

7-16 The angle of a man examined because his infant son was diagnosed as having bilateral Axenfeld anomaly with severe secondary glaucoma. The family history is otherwise negative for Axenfeld anomaly and for glaucoma. This man had one small area of prominent Schwalbe’s line with iris adhesions. In other respects, both eyes were normal and the intraocular pressures were normal.
Rieger anomaly demonstrates all of the changes seen in Axenfeld anomaly with the addition of abnormalities of the iris. The iris is thinned, with areas of atrophy and hole formation (7‑17). This leads to polycoria (multiple pupils) (7‑18) and corectopia (a displaced pupil) (7‑19). Hole formation generally occurs 180° from the direction of the displaced pupil. Occasionally the changes to the iris can be progressive (Judisch et al, 1979, and Honkanen et al, 2006).
In Rieger syndrome the ocular changes of Rieger anomaly are associated with systemic abnormalities. The most common abnormalities include maxillary hypoplasia (7‑20), small and missing teeth (microdontia and hypodonitia) (7‑21), redundant periumbilical skin (7‑22), and hypospadius.
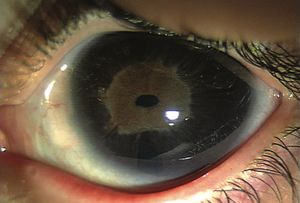
7-17 Marked iris hypoplasia in Rieger anomaly.
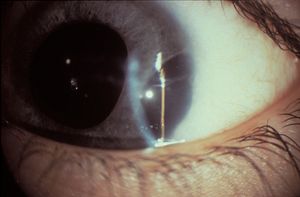
7-18 Polycoria in Rieger anomaly.
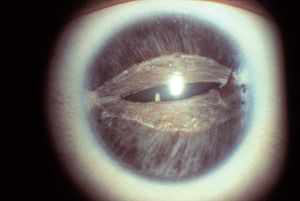
7-19 Corectopia in Rieger syndrome. The pupil has been stretched horizontally. The superior and interior iris are thin. The same patient is shown in 7‑20 to 7‑22. (Reprinted with permission. Arch Ophthalmol. 1979;7:2120–2122.Copyright © 1979, American Medical Association. All Rights Reserved.)
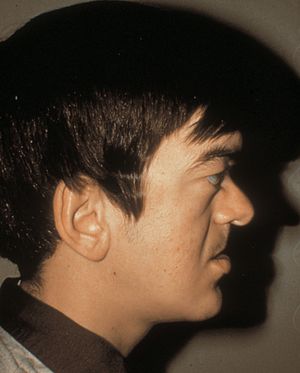
7-20 Rieger syndrome, showing poorly developed maxilla with flat face (same patient as in 7‑19, 7‑21, and 7‑22). (Reprinted with permission. Arch Ophthalmol. 1979;7:2120– 2122.Copyright © 1979, American Medical Association. All Rights Reserved.)
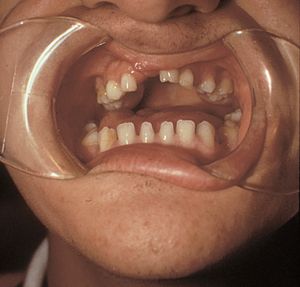
7-21 Rieger syndrome showing microdontia and hypodontia (same patient as in 7‑19, 7‑20 and 7‑22). (Reprinted with permission. Arch Ophthalmol. 1979;7:2120–2122.Copyright © 1979, American Medical Association. All Rights Reserved.)
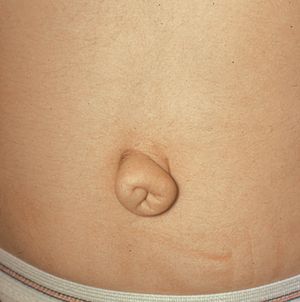
7-22 Redundant periumbilical skin in Rieger syndrome (same patient as in 7‑19 to 7‑21). (Reprinted with permission. Arch Ophthalmol. 1979;7:2120–2122.Copyright © 1979, American Medical Association. All Rights Reserved.)
Aniridia
Although aniridic patients usually appear to have no iris on examination with a slit lamp, some iris tissue is always present. In general the iris stump is so small that it can be seen only gonioscopically (7‑23, 7‑24) or histopathologically (7‑25). Because of corneal and lenticular disease, gonioscopy can be difficult, but a clear view into an aniridic angle often provides a view of the angle structures, ciliary processes, and even peripheral retina (7‑26).
Aniridia shows many variations. Most patients also have corneal pannus (7‑27), nystagmus, cataract (7‑28), foveal hypoplasia (7‑29), and poor vision. The disease is usually autosomal dominant but may be autosomal recessive or sporadic. Mutations in the PAX6 gene are associated with aniridia (Jordan et al, 1992). The autosomal recessive form may be associated with mental retardation. The sporadic form may be associated with Wilms tumor. Some aniridic families have relatively normal visual acuity (Elsas et al, 1977) but most have poor acuity at birth due to foveal hypoplasia, or gradual vision loss associated with progressive corneal pannus, cataract, and glaucoma.
Glaucoma develops in approximately 50% of patients with aniridia, usually in late childhood or early adulthood. The angle is typically open at birth and closes over time. The mechanism of glaucoma development is thought to be rotation of the iris stump over the trabecular meshwork. This may be due to the contraction of strands of iris that extend to the trabecular meshwork from the root of the iris (Grant and Walton, 1974).
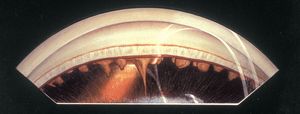
7-23 Inferior angle in aniridia demonstrating a small iris stump and a pale trabecular meshwork. The eye had undergone a previous cataract extraction. Some opaque lens material remains at the bottom of the illustration. The peripheral fundus is visible.
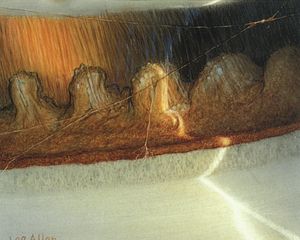
7-24 Superior angle in aniridia demonstrating a small stump of iris that covers the scleral spur and part of the posterior trabecular meshwork. The trabecular meshwork has no pigmentation and is identified by the corneal wedge. The patient is aphakic. The peripheral fundus is visible.
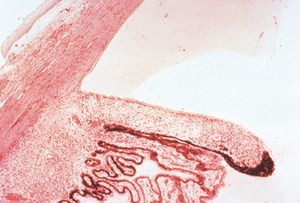
7-25 Histopathology of aniridia. The iris is very small and barely extends past the ciliary processes. (Courtesy of the National Museum of Health and Medicine, Armed Forces Institute of Pathology.)
7-26 Angle of a patient with aniridia, showing that the peripheral retina can be viewed gonioscopically.
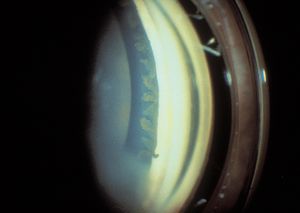
7-27 Phakic patient with aniridia. Corneal pannus obscures the view of the angle in the inferior portion of the photograph.
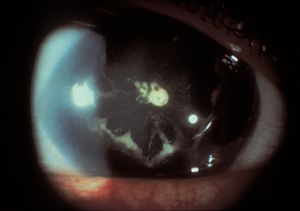
7-28 Cataract in a patient with aniridia (same patient as in 7‑27).
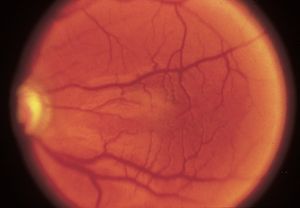
7-29 Hypoplastic macula in a patient with aniridia. (Courtesy of Elizabeth A. Hodapp, MD, Bascom Palmer Eye Institute.)
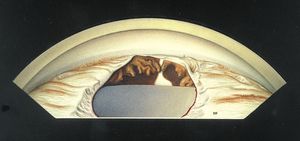
7-30 Inferior angle demonstrating an iris coloboma. The ciliary body is also absent in this region, allowing the sclera to be seen. The absence of zonules in this region has caused a focal flattening of the lens.
Coloboma
The rare condition of iris coloboma is usually found inferiorly in an eye that is otherwise normal (7‑30). It represents a failure of closure of the embryonic cleft. An iris coloboma in isolation is not typically associated with glaucoma.