Authors: Sumit Garg, MD; Ann Z McColgin, MD; Roger F. Steinert, MD
Introduction
The argon fluoride excimer laser, emitting ultrashort pulses at 193 nm, precisely etches the cornea with only submicron damage to the adjacent nonablated stroma. The freshly ablated surface supports rapid and stable re-epithelialization in advance of reformation of normal basal lamina complexes. Therefore, the cornea can be recontoured by the excimer laser in vivo. The dominant clinical interest in this laser has been for modification of the corneal optics, particularly for reshaping the cornea for the correction of refractive error. Superficial corneal opacities and irregularities also are treated by the laser as a therapeutic intervention termed phototherapeutic keratectomy (PTK). This chapter reviews the general principles, indications for, and clinical results of PTK.
Principles of the Excimer Laser
The term excimer is a contraction of excited dimer. Excited dimers are molecules with little or no binding in the electron ground state but a more closely bound upper energy state. Rare gas atoms interact with a halogen molecule when stimulated to the upper state by electrical discharge within the laser cavity. High-power ultraviolet (UV) radiation is emitted as the bound upper state rapidly dissociates to the ground state. Developed in 1975, the excimer laser is used scientifically to perform research in physical chemistry and pump dye lasers, and industrially to etch materials. Excimer laser emission has short pulses, typically around 10 nsec, with a repetition rate of 1 to 50 Hz. Table 1 lists the UV wavelengths obtained from common gas mixtures.
Table 1. Excimer laser emission.
|
Gas Medium
|
Wavelength (nm)
|
|
F2
|
157
|
|
Xe2
|
170
|
|
ArF
|
193
|
|
KrCl
|
222
|
|
KrF
|
248
|
|
XeCl
|
308
|
|
XeF
|
351
|
Photobiology of Excimer Laser Corneal Ablation
Research in the early 1980s showed that excimer laser-generated UV light can precisely etch a variety of polymers. Srinivasan and Leigh observed that the irradiated molecules are broken into small fragments that are ejected into the surrounding atmosphere, a process called ablative photodecomposition. Ablative photodecomposition of organic polymers is attributed to the polymer's high absorption of short UV radiation, confining the effect to near the surface, and the high energy of each UV photon. At 193 nm, a single UV photon has an energy of 6.4 eV, which exceeds the covalent bond strength of many molecules. After bond breakage occurs, intense pressure in a confined volume ejects the molecular fragments into the surrounding atmosphere.
Direct bond breakage by a high-energy photon is a photochemical laser–material interaction. The relative contribution of photochemical and thermal mechanisms to the UV ablation of organic polymers is negligible. At the shortest laser wavelengths, such as 193 nm, the high photon energy may result in a purely photochemical process of ablative photodecomposition. At longer wavelengths, absorbed photon energy leads to a local rise in temperature, causing etching through a photothermal process. At longer wavelengths, protein coagulation may occur adjacent to the ablation zone. Short laser pulses delivered at a low repetition rate help to limit local heating.
Photochemical and photothermal effects of excimer laser wavelengths on the cornea result from absorption by solid elements. Water poorly absorbs wavelengths between 193 and 293 nm. The carbon-nitrogen peptide bond is believed to be the source of a strong protein absorption peak at approximately 190 nm. Most of the corneal amino acids are nonaromatic and account for collagen absorption that begins to rise at wavelengths of less than 260 nm and particularly less than 240 nm. Aromatic amino acids absorb more strongly at wavelengths of greater than 240 nm. A collagen absorption peak around 250 nm is attributed to a carbon-nitrogen enolized peptide linkage. The corneal glycosaminoglycans have similar absorption spectra, with peaks around 190 nm and minimal absorption at 248 nm. Nucleic acids are limited to the occasional keratocyte in the stroma but are more important chromophores in the epithelium, with strong absorption at both 248 and 193 nm. Ascorbic acid, particularly concentrated in epithelial cells, has more absorption at 248 nm than at 193 nm.
Mutagenesis and carcinogenesis are concerns with UV radiation. Almost all carcinogens have been shown to be mutagens. UV radiation-induced mutation parallels absorption by DNA. The low density of stromal keratocytes offers some protection against carcinogenesis resulting from stromal photoablation. In several studies, 193-nm irradiation did not cause mutagenic or carcinogenic cellular events. Nuss et al. examined unscheduled DNA synthesis, a measure of repair of pyrimidine dimers. Compared with a control incision made with a diamond knife, unscheduled DNA synthesis did not increase after 193-nm linear ablation; in contrast, a statistically significant increase occurred after 248-nm irradiation.
Mechanisms for decreased toxicity at 193 nm include absorption of that wavelength by protein surrounding the nucleus (protein shield), lack of cytotoxicity of DNA photoproducts produced by 193-nm light, DNA damage readily repaired by the cells, or such damage that mutagenic repair processes are not possible. In a skin model, DNA damage and subsequent cytotoxicity were least at 193 nm, intermediate at 308 nm, and greatest at 248 nm.
Corneal irradiation at 193 nm also results in fluorescence between 295 and 425 nm. These emissions could be both mutagenic and cataractogenic; however, the highly attenuated energy of the fluorescence may not reach toxic levels.
Laser-Cornea Interaction
In 1983, Trokel et al. first reported the controlled etching of the cornea by an argon–fluorine (ArF) excimer laser. Puliafito and colleagues et al. compared the histopathologic effects of linear cornea ablation at 193 and 248 nm. Both studies found excellent preservation of normal corneal stromal microstructure adjacent to the ablation zone at 193 nm (Figure 1). The adjacent cornea remained optically clear. High-power transmission electron microscopy showed a submicron zone of electron density immediately adjacent to the ablation.
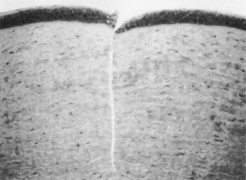
Figure 1. Light micrograph of a 193-nm, slit-like ablation in a bovine cornea. Dosage parameters: 20,000 pulses, 50 Hz, 125 mJ/cm per pulse, with a 10-νm mask (original magnification × 214). Puliafito CA, Steinert RF, Deutsch TF, et al: Excimer laser ablation of the cornea and lens: Experimental studies. Ophthalmology. 92:741, 1985.
Kerr-Muir et al. first described a pseudomembrane that appeared to seal cells and cellular nuclei transected by the laser beam. In contrast, at 248 nm, disorganization of the collagen microstructure extended into the adjacent stroma for more than 10 νm. The cornea immediately adjacent to the ablation showed a loss of transparency, indicative of thermal injury. Peyman et al. showed a significant coagulative effect from 308-nm excimer laser radiation with induced corneal necrosis, stromal opacification, and endothelial cell damage.
Krueger et al. quantitated ablation rates. Etch depth per pulse plotted against radiant energy density generated sigmoid curves. The steep portion of the curve is approximately logarithmic; this finding corresponds to observations in simpler organic polymers. The inflection point of the curve is approximately 200 mJ/cm for 193 nm, which represents the energy density yielding the most efficient ablation.
Puliafito et al. postulated that ablation at the inflection points may be clinically undesirable; at the inflection point small changes in laser output could dramatically alter the ablation rate. Ablation at higher energy densities, such as 400 to 600 mJ/cm per pulse, where the ablation rate has the flattest slope, might be preferable in situations where ablation rate is critical.
Krueger et al. observed that for wavelengths higher than 193 nm, the threshold for corneal ablation increases as the laser repetition rate decreases. At 193 nm, the threshold was constant despite varying repetition rates. This is consistent with the photochemical theory of excimer corneal ablation, in which the buildup of heat does not play an important role at 193 nm and thermal mechanisms of ablation are important for ablation at longer wavelengths.
The effect of radiation density on corneal smoothness remains controversial. Fantes and Waring showed improved corneal surface uniformity with their scanning system at higher laser energy density, especially with 500 mJ/cm per pulse. In contrast, Berns and et al. observed that such high levels of energy can be associated with corneal surface ridges, endothelial damage, and small pit formation on the stromal surface inside the linear ablation zones.
Endothelial toxicity probably is minimal, although endothelial effects can be seen despite the nearly complete absorption of 193 nm photons within 1 micron of tissue. Dehm et al. found that in linear ablations, 193-nm incisions to 90% of corneal depth produced endothelial membrane disruption similar to that seen underlying diamond knife incisions at the same depth. Frank loss of endothelial cells underlying the incision was observed at 248 nm.
Hanna et al. observed the appearance of electron-dense granules in the endothelium after surface ablation of rabbit corneas at 193 nm. These electron-dense granules migrated through Descemet's membrane over several weeks and then dissipated. The clinical significance of this observation is unknown but may be related to transient pressure injury to the endothelium. While ablating the superficial stroma, Zabel et al. measured pressures as high as 100 atm at the endothelium during ablation of the superficial stroma, but no frank disruption of the corneal endothelium occurred.
The fragments ejected from the corneal surface are visible several hundred nanoseconds after the laser exposure. For 193-nm irradiation, the particles travel at an initial velocity of approximately 400 m/sec for the first 500 nsec, but then rapidly decelerate. Particle ejection ends within 5 to 15 νsec as the decelerating particles form a mushroom-shaped cloud (Figure 2). Increased exposure energy leads to increases in both the plume size and initial velocity of the ejected fragments. Analysis of the plume has identified numerous molecules that contain between 10 and 20 carbon atoms.

Figure 2. Ablation plume created by 193-nm excimer pulse at 900 mJ/cm per pulse. High-speed photograph obtained by illumination with Nd:YAG laser at 532 nm 50 msec after the excimer pulse struck the corneal surface (original magnification × 4.5). Puliafito CA, Stern D, Krueger RR, et al: High-speed photography of the excimer laser ablation of the cornea. Arch Ophthalmol. 105:1255, 1987.
Therapeutic Applications of the Excimer Laser
The excimer laser has received attention for its ability to reshape the corneal surface to correct ametropia. Although correction of postoperative refractive errors and anisometropia has been called therapeutic, PTK typically refers to the use of the excimer laser for treatment of other corneal disease.
This section discusses the application of excimer laser ablation for recurrent erosions, treatment of surface irregularities, removal of superficial opacities, and treatment of complications after photorefractive keratectomy (PRK) and laser in situ keratomileusis (LASIK). PTK has been used successfully in pediatric and adult diseases.
Recurrent Corneal Erosions
Painful recurrent erosion syndrome, whether resulting from trauma or anterior membrane dystrophy (Cogan's dystrophy or map-dot-fingerprint dystrophy), results from abnormalities in the epithelial basement membrane complex. These patients usually respond to topical lubrication therapy, hyperosmotics, bandage soft contact lenses, debridement of the epithelium and basement membrane, or anterior stromal micropuncture. Occasionally patients continue to have painful recurrent erosions despite these measures, and superficial PTK can be curative in this setting.
The ablated anterior corneal surface appears highly supportive of stable re-epithelialization. Although completely normal reformation of the basal lamina complex, including normal density of hemidesmosomes and anchoring fibrils, may take months to years, the rapid and stable re-epithelialization that occurs after ablation, with absence of punctate keratitis, staining defects, or symptoms of recurrent erosion, is impressive.
The objective of PTK in the treatment of recurrent erosions is to remove enough of the superficial Bowman's layer to permit formation of a new basement membrane with adhesion structures; therefore, it is the most superficial of the PTK procedures. The usual technique is to debride the loose epithelium manually. If the area of clinical recurrent erosion is localized, the surgeon may find that the epithelium is poorly adherent over most of or all the corneal surface. With the use of a large spot size, such as 6.5 mm, the surgeon ablates the central superficial corneal surface.
Varying techniques are used to expose the peripheral cornea outside the central zone. With the VISX laser, a smaller spot is manually scanned around the periphery by moving the patient's head. Some smaller spot scanning lasers can be programmed so the scan will include the periphery as part of areas treated. In a retrospective analysis of ten consecutive eyes van Westenbrugge showed small spot PTK using the NIDEK EC-5000 excimer laser to have an 80% success rate.
The amount of tissue ablated to maximize the effect with minimal refractive change has not been elucidated. Some investigators remove only 2 to 6 microns, but others have found that removal of 10 microns results in fewer recurrences. After the laser ablation, a drop of topical antibiotic, steroid, and sometimes a nonsteroidal agent is applied, followed by placement of a bandage contact lens. Follow-up is required until epithelialization is complete, then the contact lens is removed. Patients typically are instructed to continue aggressive lubrication, including nightly application of lubricating ointment, for several months after the procedure.
Clinical studies of PTK for recurrent erosions have been encouraging. Morad et al. treated 23 eyes for recurrent or persistent corneal erosion with PTK by ablating 2 to 6 microns of tissue after epithelial removal. Eighty-three percent of patients remained were symptom-free during the 12- to 60-month follow-up, and none experienced a significant refractive change.
Cavanaugh et al treated 36 eyes for recurrent erosion in the setting of anterior basement membrane dystrophy, and found that 86.6% had no recurrence of symptoms after 12 months of follow-up. The average refraction change was +0.72 +\- 1.26 diopters (D), and a positive correlation was found between the number of pulses applied and induced refractive change.
Jain and Austin reported that of 77 eyes treated with PTK for recurrent erosions, 69% were symptom-free after 6 to 55 months of follow-up and 100% were free of acute episodes after 1 or 2 treatments. Patients treated for recurrent erosions resulting from trauma had a better outcome than those whose symptoms were idiopathic or caused by a basement membrane dystrophy. Higher success rates with PTK in recurrent erosion syndrome due to trauma compared to corneal dystrophies have been shown. Other studies have shown a 60% to 100% cure rate with one or more PTK treatments for recurrent erosions. Quantitative studies indicate that the fundamental health of the corneal epithelium may be improved after PTK.
More studies are necessary to determine the optimal amount of tissue removal to minimize both recurrences and significant refractive changes. Sridhar et al. found that diamond burr polishing was at least as effective although simpler and less costly than PTK for recurrent erosions. Another study concluded that intraepithelial PTK and alcohol delamination of the epithelium were both effective for trauma-related recurrent erosions where ablation of Bowman's layer or anterior stroma does not seem necessary.
PTK has successfully reduced painful bullae in eyes with pseudophakic bullous keratopathy, and when combined with amniotic membrane grafting, may decrease the recurrence rate.
Irregular Surface
Surface irregularities can severely disrupt the optical performance of the anterior cornea. Patients may complain of diminished visual acuity and optical aberrations such as monocular diplopia, glare, or halos. Common causes of surface irregularities include
- Anterior corneal dystrophies, including basement membrane dystrophy, Reis-Bucklers' dystrophy, Schnyder crystalline corneal dystrophy, and anterior granular and lattice stromal dystrophies;
- Elevated corneal scars, including apical scars associated with keratoconus; and
- Degenerations, such as Salzmann nodule formation and band keratopathy. Irregular astigmatism also can occur after refractive surgery.
Many sources of surface irregularity are also associated with corneal opacities. Smoothing the optical surface alone sometimes significantly improves the patient's visual function without the hyperopic shift and potential scarring that can be associated with the deeper ablations often necessary for removal of opacities.
Hard contact lens refraction can be helpful in determining what component of a patient's visual complaints is secondary to surface irregularity alone. Improvement in visual acuity with a hard contact lens is attributable to irregularity, and the remaining vision impairment with the hard contact lens in place is caused by the opacity.
Irregularities are not intrinsically removed by exposure to repeated pulses of the excimer beam. Photons that encounter an irregular surface do not discriminate between elevations and depressions, and tissue that is removed largely replicates the original irregularity (Figure 3). Strategies have been devised to protect the deeper tissues (valleys) while exposing elevated pathology (peaks) to the ablating photons.
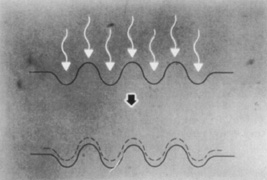
Figure 3. An irregular corneal surface ablated without any masking fluid will maintain the irregular contour as the ablation continues into the deeper stroma. Kornmehl EW, Steinert RF, Puliafito CA: A comparison study of masking fluids for excimer laser phototherapeutic keratectomy. Arch Ophthalmol. 109:860, 1991.
Epithelial hyperplasia commonly occurs in areas of corneal depression. Performing transepithelial ablation, where the thickened epithelium shields the valleys from the laser energy, can take advantage of the natural masking effect of the epithelium.
One strategy for removing surface irregularities of Reis-Bucklers' dystrophy involves ablating through the epithelium that is intermixed with the abnormal substance until the epithelium is largely absent. This absence is determined by the disappearance of the fluorescence that occurs during epithelial ablation. Further ablation is then performed in conjunction with masking fluids.
Commonly used masking fluids are artificial tear substances, which are available in varying viscosity. Kornmehl et al. compared artificial tear substances of varying viscosity with saline and a nonfluid control. In a model in which the corneal surface initially was roughened with sandpaper, solutions of moderate viscosity yielded a smoother surface than more viscous artificial tears, and markedly better results than the nonviscous saline solution. All fluids outperformed the nonfluid control. A subsequent study found good results with a preparation of 0.25% sodium hyaluronate.
Figure 4 shows the theoretic concept that a fluid with inadequate viscosity tends to run off, providing insufficient protection for the valleys, whereas a substance that is too viscous drapes over the peaks, protecting the elevations, where ablation is desired.

Figure 4. (A) Ablation of an irregular surface with a fluid of inadequate viscosity will not adequately protect the valley in which runoff can occur, and irregularities will persist as ablation progresses. (B) Ablation of an irregular surface with a highly viscous fluid will be irregular because of the variable coating of different thicknesses of the fluid. (C) The ideal fluid has adequate ultraviolet absorption and moderate viscosity to cause ablation of the exposed peaks while masking the underlying valleys. Kornmehl EW, Steinert RF, Puliafito CA: A comparison study of masking fluids for excimer laser phototherapeutic keratectomy. Arch Ophthalmol. 109:860, 1991.
In addition to to the effect of viscosity, different solutions may have varying effectiveness in the absorption of 193-nm photons. A thin film of fluid must have adequate absorption to prevent ablation of the underlying tissue. Enhancing absorption at 193 nm may contribute to a more effective masking fluid for phototherapeutic keratectomy of an irregular surface.
A gel-like substance also could be applied uniformly over the cornea and molded into a new, smooth anterior surface. This gel would be ablated and would expose the peaks while protecting the valleys without the need for repeated application of viscous fluids. To be fully effective, the ablation rate of the gel would have to match that of the pathologic tissue. Such a gel has yet to be implemented in clinical practice.
Superficial Corneal Opacities
Previously, the only options for removal of corneal opacities were lamellar or penetrating keratoplasty. The excimer laser can remove some superficial opacities without the need for more invasive surgery. Figures 5 and 6 show examples of corneal opacities treated with PTK, resulting in marked improvement in central corneal clarity.
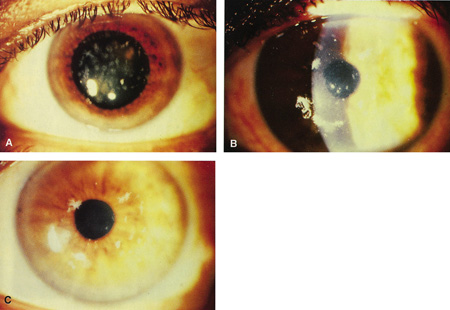
Figure 5. (A) Preoperative appearance of a patient with the granular appearance of the Avellino variant of lattice dystrophy. Most of the opacities are superficial. (B) Four days after excimer ablation, re-epithelialization has occurred. Most of the opacities are eliminated from the treatment zone. (C) One month after surgery, dramatic improvement in central corneal clarity has occurred. Steinert RF: Therapeutic keratectomy: Corneal smoothing. In Thompson FB, McDonnell PJ [eds]. Color Atlas and Text of Excimer Laser Surgery. New York: Igaku-Shoin, 1993:179.
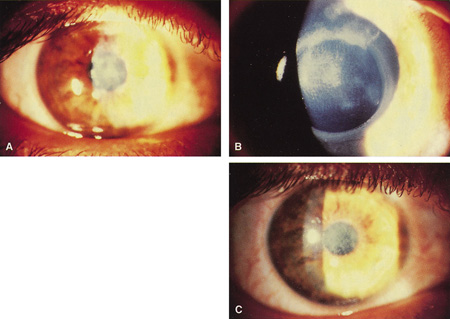
Figure 6. (A) Herpetic anterior stromal scar before excimer phototherapeutic keratectomy. (B) Appearance immediately after excimer phototherapeutic keratectomy, with reduction of the stromal scarring. The edge of the 4.5-mm–round ablation zone is evident. (C) Three months after surgery, an oblique slit illumination shows some persistence of the deeper herpetic scarring plus the addition of reactive reticular haze. At this time, however, acuity was 20/30 versus 20/80 before surgery.
Maloney et al. studied 232 patients treated with PTK for corneal visual loss (resulting from opacities, surface irregularity, or both). Approximately 45% of patients at each follow-up visit experienced a two-line improvement of best-corrected vision. Other studies have revealed similar results. A German group used PTK to treat dense superficial corneal scars in a small cohort of children increasing corneal transparency and visual acuity.
When evaluating a visually significant corneal opacity, it is critical to assess its depth. In some conditions, such as Reis-Bucklers' dystrophy, the location of the abnormality is limited to the anterior stroma. In lattice or granular dystrophy, optically significant opacities in the visual zone must be examined and their depth assessed.
Post-inflammatory scarring is more difficult to assess because the posterior extent of the opacity typically is typically ill defined, and often deeper than initially appreciated at slit-lamp examination. Partial removal of the opacity may be adequate for the patient's visual needs. An optical pachymeter can be useful in measuring the depth of the opacity, although the opacity itself may preclude its accurate use. Advances in anterior segment optical coherent tomography (AS-OCT) have allowed improved evaluation of the depth of involvement of pathology.
AS-OCT can be useful in surgical planning by providing accurate dimensions of the pathology, which can guide the number of pulses applied by the excimer laser. AS-OCT can simulate PTK in cases of stromal dystrophies, allowing the surgeon to choose the depth of ablation, which could minimize undesired refractive side effects.
Visual impairment associated with these disorders often results from a combination of the opacity itself and accompanying corneal irregularity, both of which must be treated to obtain an optimal visual result.
Depending on its cause, the opacity may ablate at a different rate than the surrounding stroma. The hyaline material in granular dystrophy ablates more slowly than the surrounding stroma. A surface that was smooth before surgery can become irregular after PTK if attention is not given to maintaining a smooth surface during the procedure.
It can be effective to debride discrete elevated opacities such as Salzmann nodules or calcium deposits manually with a blade before PTK and then use masking agents to expose the elevated remaining irregularities during the ablation to create or maintain a smooth optical surface.
Ablation usually is performed as a planar disc ablation alone or followed by a hyperopic ablation. These approaches are intended to reduce the hyperopic shift. Although the initial ablation may be planar and may retain the original anterior corneal curvature, remodeling after laser treatment with new collagen formation and epithelial hyperplasia at the periphery of the treatment zone may result in net flattening and a hyperopic shift of up to +8.00 D in some cases.
Amm and Duncker reported that, of 45 patients treated, all refractions remained stable after PTK for recurrent erosions, whereas after treatment for corneal scars, anterior stromal dystrophies, or surface irregularities, 40.6% of patients developed a hyperopic shift, 9% developed a myopic shift, and 40.6% remained stable. Deeper ablations were associated with a greater likelihood of hyperopic shift.
Katsube et al. proposed that the fluid-filled porous nature of the cornea accounts for much of the tendency for the hyperopic shift after PTK.
In assessing suitability for PTK, refractive status is a major component. Unless the patient is moderately myopic before surgery, and either has bilateral disease or readily accepts contact lens correction in the fellow eye, the potential for unacceptable anisometropia is high. However, postoperative use of a hard contact lens often is of additional benefit by optically eliminating residual irregular and regular astigmatism.
In addition to the hyperopic shift, deeper ablation, particularly exceeding 50 to 100 µm, usually is accompanied by haze that can become visually significant at its extreme.
It is unclear whether intensive steroid administration after laser treatment significantly retards scarring, and to what extent intensive steroid treatment leads to a more pronounced permanent hyperopic shift by retarding wound healing.
Briefly applying topical mitomycin-C (MMC) immediately after the ablation may reduce scar formation. MMC used prophylactically has been shown to prevent significant recurrent haze in eyes undergoing PTK retreatment.
Although PTK may reduce the optical and surface disruption of anterior dystrophies, most dystrophies recur naturally, requiring a repeat PTK or transplantation.
Miller et al. reported a case of Reis-Bucklers dystrophy that experienced 2 rapid recurrences after conventional PTK, but had 1 year without evidence of recurrence after a third PTK in conjunction with topical application of MMC.
Ayres et al. noted prevention of recurrence in areas treated with MMC, but recurrence outside of the treated area. MMC should be used with caution, however, as it has been associated with corneal and scleral melts, cataract formation, and endothelial cell loss.
Elsahn et al. described using PTK for the treatment of subepithelial nodules in keratoconus patients who were intolerant to contact lenses. They found that PTK could be used to treat the nodules and improve contact lens tolerance delaying possible penetrating keratoplasty.
Taenaka et al. have shown PTK to be therapeutic for the treatment of acanthamoeba keratitis not responsive to medical therapy or corneal debridement. They report using a combination of PTK followed by limited keratectomy to remove the majority of infected tissue. Anti-amoebic therapy was continued for several weeks. The patient had no recurrences for the 33-month follow-up period, with final best-corrected visual acuity of 20/20. This novel idea allows for a smooth removal of stromal lesions, resulting in superior postoperative visual acuity.
PTK does not impair the outcome of subsequent penetrating keratoplasty when surgical intervention is needed.
Treatment of PRK and LASIK Complications
The major interest in the excimer laser has been in the alteration of the anterior corneal contour for the correction of refractive errors. Improvements in the techniques and instrumentation for PRK and LASIK have minimized the risk of significant visual loss associated with these procedures. However, irregular astigmatism or scarring secondary to the ablation, the patient's healing response, or flap complications can still occur.
Epithelial hyperplasia and hypoplasia (Figure 7) often minimize the irregularities over time, and superficial haze after PRK often slowly fades. In some cases, loss of best-corrected vision, multiplopia, or other optical aberrations can persist and need to be managed surgically. PTK can be helpful in the management of these complications.
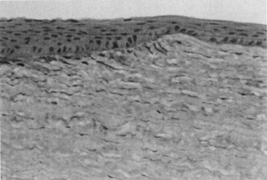
Figure 7. Normal thickness of the epithelium is seen over the untreated cornea (right). Hyperplasia of the epithelium in the ablation zone (left) approximately doubles the original corneal thickness, leading to restoration of a smooth corneal contour (original magnification × 160).
Central Islands
Central islands, usually defined as at least 1.00 D of central steepening in the presence of overall flattening on corneal topography, are often evident after PRK and less commonly after LASIK (Figure 8). Patients with symptomatic central islands may experience mild loss of best-corrected visual acuity, multiplopia, ghost images, halos, glare, and increased astigmatism. The refraction usually is mildly myopic because of the central steep zone.
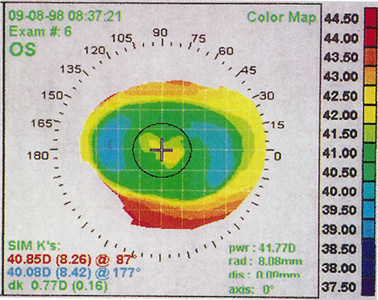
Figure 8. Corneal topography of a central island after a toric ablation. Note central steepening within the otherwise flattened ablation zone.
Preventing central island formation requires adherence to the manufacturer's recommendations. These include "anti-island" ablation treatments where extra pulses are delivered to the center of the ablation zone. Treatment may be interrupted several times to dry the central stroma with a cellulose surgical sponge.
Most central islands resolve or become optically insignificant by 6 months after PRK surgery. Histologic documentation of the healing mechanism of central islands remains unavailable. A common hypothesis is that the central island represents unablated stromal tissue, and that the "healing" consists of a thickening of the epithelium in the midperiphery surrounding the island, which gradually envelops and covers the island.
In patients with symptomatic central islands after PRK, it is prudent to wait at least 6 months before considering retreatment. Some topographically evident central islands do not cause symptoms and should be ignored. When symptoms are present and unchanged after 6 months, and the topography and refractive status are stable, treatment should be considered.
After LASIK , in contrast, surface remodeling is minimal. If a symptomatic central island is stable for at least 2 months, relifting the flap and treating the central island in the stromal bed can be considered.
Two treatment options for central islands after PRK have been advocated. In the first treatment option, the central epithelium is removed with a blade, exposing the central stromal island. The peak of the island is then treated with either a PRK algorithm whose optical zone is set to the diameter of the central island and whose dioptric correction matches the height of the island or a PTK algorithm set to the width of the island and the number of pulses calculated to create the dioptric flattening needed (approximately 12 pulses per diopter). In either case, the dioptric correction is based on the height of the island as detected on an early topography, usually the 1-month topographic analysis. By 3 to 6 months, the island may appear to be less elevated due to partial hyperplasia of the surrounding epithelium; therefore, retreatment will be incomplete.
The second treatment option is to retain the epithelium and use a PTK disc ablation equal to the largest diameter of the island. The first area of stroma to be exposed is at the peak of the island if epithelial hyperplasia has occurred around the central island. This can be visualized as the "breakthrough" of the cobalt blue fluorescence that occurs with ablation of the epithelium; fluorescence readily visualized when the room lights and microscope lights are shut off. Ablation through the epithelium progressively exposes the island, whereas the thicker peripheral epithelium shields the surrounding stroma. When the central nonfluorescent stromal island reaches the width of the topographic island as it was seen at 1 month, the island ablation is complete. If the breakthrough of the transepithelial ablation does not occur centrally but rather simultaneously throughout the optical zone, PRK or PTK pulses are employed, calculated as previously described.
For both techniques, after removal of the tissue island, aggressive topical steroids should be used until stable healing and stable refractive status are achieved to discourage any central scarring.
After LASIK, central islands are treated by lifting the pre-existing flap and ablating the central stroma with a diameter and depth corresponding to the width and height of the island on the topographic map. Preference is to program a myopic correction for the dioptric value of the height of the island on topography. The width is programmed to match the topographic diameter of the island.
Food and Drug Administration restrictions in the United States may not allow the surgeon to select a small optical zone in myopic corrections, the surgeon using a broad-beam laser can either manually stop the exposure when the ablation is observed to reach the desired size, or more precisely, apply the number of pulses that correspond to a certain diameter. The laser manufacturer can supply a nomogram interrelating dioptric correction, diameter, and number of pulses.
Corneal Haze After PRK
After PRK, subepithelial corneal haze usually develops in several weeks, peaks within 1 to 2 months, and then resolves spontaneously during the next 6 to 12 months (Figure 9). Haze is more prominent in eyes after treating higher degrees of correction, especially when using smaller ablation zones. Haze is often asymptomatic, being more noticeable to the examiner than the patient. Haze can contribute to loss of contrast sensitivity or even loss of best-corrected visual acuity.
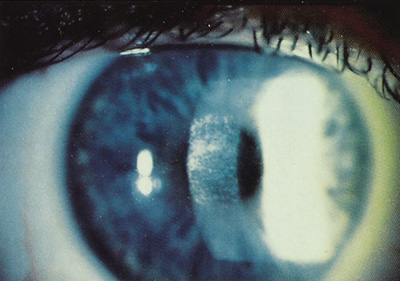
Figure 9. Moderate central corneal haze 3 months after photorefractive keratectomy.
Significant subepithelial haze may improve with increasing the frequency of topical steroids. If visually significant haze persists without improvement after 6 to 12 months, surgical intervention may be indicated. Often haze formation is accompanied by regression, so that repeat excimer ablation can simultaneously treat both the regression and the haze. Retreatment in patients with significant haze yields less predictable results than retreatment of patients with regression in the absence of haze.
Seiler et al. used a combined PTK-PRK approach to treat 21 undercorrected eyes with corneal haze and found scar recurrence in 4 eyes (19%).
Pop and Aras found that patients with greater haze had a 40% rate of overcorrection. Retreatment should be directed toward decreasing haze to a clinically insignificant level rather than eliminating it.
A transepithelial approach often is used in PTK mode with a large ablation diameter. With the PRK mode, it has been recommended to correct only 50% to 60% of the myopia to avoid overcorrection. The use of MMC has been shown to decrease the amount of haze. PTK has been successfully combined with amniotic membrane transplants in two cases of severe haze following PRK.
Decentered Ablations
Decentered ablations can occur with PRK or LASIK secondary to initial improper positioning, or loss of patient fixation with drift of an initially centered treatment (Figures 10 and 11). During the ablation, the patient's head must be stabilized, typically by the surgeon's hands, and the patient and surgeon must monitor the fixation beams.
Decentered ablations are more common after correction of higher degrees of refractive error, probably secondary to the longer fixation time required. They are more likely to be symptomatic with the use of smaller optical zones, because the edge of the ablation is then more likely to be within the pupillary margin. Mild decentrations may be asymptomatic, but if greater than 1 mm, are often associated with glare, halos, monocular diplopia, and occasionally loss of best-corrected visual acuity.
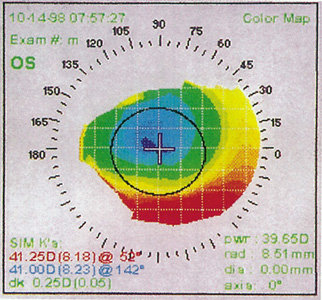
Figure 10. Corneal topography of a decentered ablation secondary to initial misalignment.
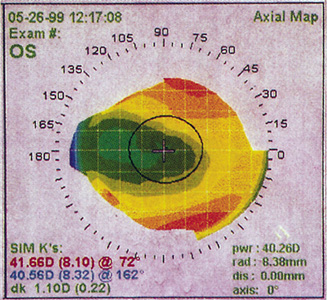
Figure 11. Corneal topography of decentered ablation secondary to drifting of the patient's fixation after an initially well-aligned treatment. The patient has 20/20 uncorrected vision in this eye but is significantly bothered by glare and halos.
Decentrations are difficult to treat; however, some success has been found using the excimer laser. In one approach, the epithelium is retained over the topographically flattened zone to act as a mask. The epithelium outside the ablation zone is removed mechanically or with the excimer laser in PTK mode.
A ridge at the border of the treatment zone can be ablated with a small optical zone PTK. A PRK treatment then is performed either centrally or decentered 180 degrees away from the first ablation, with the optical power guided by topography.
The already flattened treatment zone is protected by the pre-existing epithelium and, if necessary, other masking agents such as high-viscosity artificial tear fluids or a methylcellulose sponge.
Pallikaris described the treatment of decentered ablations without the use of the laser by using a limbal relaxing incision or arcuate keratotomy oriented 180 degrees opposite the decentration. Studies are necessary to determine whether these incisions result in better outcomes compared with retreatment with the excimer laser.
Decentration usually results in large amount of the high order aberration known as coma. With the clinical introduction of wavefront-guided optics for primary treatment, therapeutic applications will likely be developed that will be a more precise and effective method for correcting decentration.
LASIK Flap Complications
When performing the keratectomy for LASIK, a flap of uniform thickness with a smooth interface is essential. When a thin, incomplete, or buttonholed flap is created, the flap should be replaced without applying the ablation. Irregular astigmatism and scarring with loss of best corrected-vision or optical aberrations still can occur and are difficult to treat. PTK potentially can be used to treat the irregular surface in these cases.
Kapadia and Wilson reported a case of a buttonholed flap that healed with superficial scarring associated with monocular diplopia and glare. Transepithelial PTK followed by PRK for partial treatment of the refractive error was performed with resolution of the optical aberrations.
A case of a buttonholed LASIK flap with epithelial ingrowth, was successfully treated with PTK along with MMC without haze formation or recurrence of epithelial ingrowth at 24 months.
Leu and Hersh used PTK on the underside of the flap and on the stromal bed to resolve a case of recurrent diffuse lamellar keratitis (DLK) unresponsive to conventional treatment; ablation of inciting toxins was hypothesized to be the basis for the resolution.
PTK can help resolve recurrent erosions and anterior basement membrane irregularities after LASIK, similar to managing primary recurrent erosions as previously discussed.
Flap Striae
Large macrofolds that occur because of flap slippage in the early postoperative period should be treated with immediate repositioning. Long-standing macrofolds, are resistant to repositioning and manual smoothing, as are microstriae that disrupt the anterior corneal optics (Figures 12 and 13).
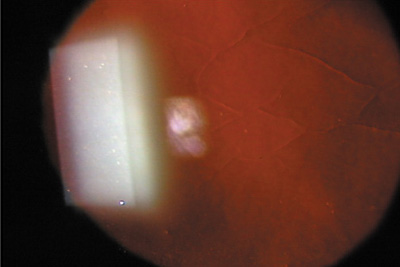
Figure 12. Slit-lamp retroillumination photomicrograph of microstriae. Reproduced with permission from Steinert RF, Ashrafzadeh A, Hersh PS: Results of phototherapeutic keratectomy in the management of flap striae after LASIK. Ophthalmology. 111:740, 2004.
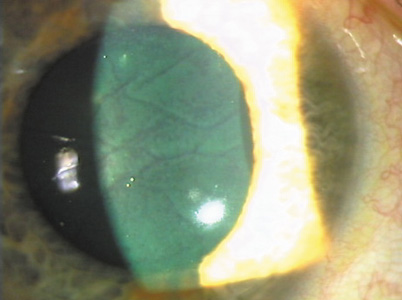
Figure 13. "Negative staining" pattern of fluorescein in the tear film because of disruption by microstriae. Reproduced with permission from Steinert RF, Ashrafzadeh A, Hersh PS: Results of phototherapeutic keratectomy in the management of flap striae after LASIK. Ophthalmology. 111:740, 2004.
Steinert et al. reported excellent results with PTK used to treat these irregularities. Figure 14 schematically shows the concept of the PTK treatment of striae. The technique, as performed with the VISX broad-beam laser, is to use the PTK mode for planar ablation with the maximal 6.5 mm zone. With the tracker engaged, 200 pulses are applied to the cornea, performing transepithelial ablation. The epithelium acts as a masking agent for the PTK. The elevated ridges are exposed first where the epithelium is thinnest whereas the valleys between the ridges, where the epithelium is hyperplastic, are protected (Figure 15).
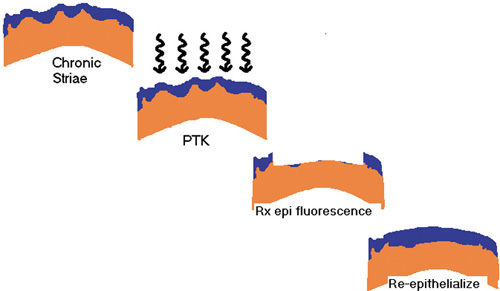
Figure 14. Schematic diagram of the reduction in optical disturbance of striae by transepithelial phototherapeutic keratectomy. Reproduced with permission from Steinert RF, Ashrafzadeh A, Hersh PS: Results of phototherapeutic keratectomy in the management of flap striae after LASIK. Ophthalmology. 111:740, 2004.
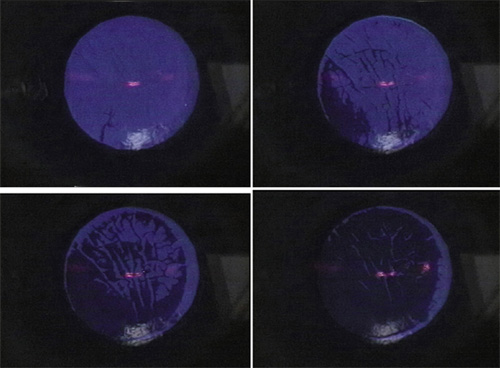
Figure 15. Sequential operative photographs of the epithelial fluorescence pattern seen during phototherapeutic keratectomy (PTK) for microstriae. (A) Initial perforation over most elevated region of striae. (B) With successive pulses, epithelial fluorescence over most elevated striae disappears. (C) With further pulses, the epithelium between the striae recedes, with further reduction in fluorescence. (D) At the end of the transepithelial phase of the PTK, most of the epithelium is removed, with only minimal residual fluorescence in the areas of thickest epithelium between the striae. Reproduced with permission from Steinert RF, Ashrafzadeh A, Hersh PS: Results of phototherapeutic keratectomy in the management of flap striae after LASIK. Ophthalmology. 111:740, 2004.
Next, the tracker is turned off, and the ring illuminator is turned on, generally revealing a large amount of striae resulting from the removal of the partially masking epithelium.
At this point, the surgeon gently debrides any remaining epithelium, stroking with a minimally moist surgical spear sponge or, if needed, a back-tilted surgical blade in the direction away from the hinge.
More laser pulses are applied in short bursts of 5 or so pulses, with a wipe of masking fluid between each burst. A medium viscosity artificial tear usually is the best choice. The cornea should look slightly moist but not with so much fluid that the striae disappear. If the masking fluid bubbles, or the laser pulses sound like a dull thud rather than a snap, too much fluid is present.
The PTK procedure stops whenever the cornea looks smoother, but in any case, no more than 100 further pulses (300 total) are applied, in order to avoid a major hyperopic shift. Used with this technique, the typical hyperopic shift is about 1 D on average. PRK can be applied after PTK to correct any significant optical error. This will be less accurate than primary PRK due to the variable effect of the PTK, however. No optically significant haze was observed in the published series. This technique resulted in stable results and minimal complications at 2 years follow-up.
Topography-Based Ablations
Despite the PTK techniques available, treatment of irregular astigmatism remains suboptimal. Fine surface irregularities can be treated using existing epithelium or masking agents to target the raised areas. Broad surface irregularities that often cause irregular astigmatism after refractive surgery or penetrating keratoplasty are not easily treated in this fashion. New technology in which topographic data are incorporated directly into laser software will be helpful for the treatment of all types of astigmatism.
Wiesinger-Jendritza et al. reported a series of 23 eyes treated with LASIK using topography-based ablations for irregular astigmatism after penetrating keratoplasty, trauma, or previous excimer laser surgery. They found that 61.9% were partially corrected and 19.4% were fully corrected after the treatment. Improvements in this technique will likely be seen as topographic analysis becomes incorporated into the laser for real-time feedback on topographic changes during the ablation.
Vinciguerra and Camesasca have reported good results with a topography-based custom therapeutic treatment of highly aberrated eyes. In 52 eyes, the percentage with best spectacle-corrected visual acuity (BSCVA) of 20/30 or better increased from a preoperative rate of 59% to 88% at one postoperative year, and BSCVA of 20/15 was reached by 25% of eyes compared to none preoperatively.
Conclusion
Phototherapeutic keratectomy is an important option for patients with painful recurrent erosions. PTK offers patients an alternative to lamellar or penetrating keratoplasty for the correction of corneal opacities and surface irregularities. Excimer lasers with real-time corneal topography feedback incorporated into the system will likely provide dramatic improvements in the ability to achieve a smooth corneal surface for patients with irregular astigmatism, invaluable in the treatment of patients with irregular astigmatism after penetrating keratoplasty and previous refractive surgery. Topography-based excimer treatments may allow the surgeon to treat regular astigmatism more precisely and reshape every corneal surface to achieve the optimal corneal contour for best visual results.
Suggested Reading
- Burlamacchi P. Laser sources. In: Hillenkamp F, Prateri R, Sacchi CA, eds. Lasers in Biology and Medicine. New York, NY: Plenum; 1980:1-16.
- Searles SK, Hart GA. Stimulated emission at 281.8 nm from XeBr. Appl Phys Lett. 1975;27(4):243-245.
- Deutsch TF, Geis MW. Self-developing UV photoresist using excimer laser exposure. J Appl Phys. 1983;54:7201
- Garrison BJ, Srinivasan R. Microscopic model for the ablative photodecomposition of polymers by far-ultraviolet radiation (193 nm). Appl Phys Lett. 1984;44:849.
- Srinivasan R. Kinetics of ablative photodecomposition of organic polymers in the far-ultraviolet (193 nm). J Vac Sci Tech. 1983;1:923.
- Slavin W. Stray light in ultraviolet, visible, and near-infrared spectrophotometry. Anal Chem. 1963;35(4):561-566.
- Smith KC. Ultraviolet radiation effects on molecules and cells. In: Smith KC, ed. The Science of Photobiology. New York, NY: Plenum; 1985:113.
- Nuss RC, Puliafito CA, Dehm EJ. Unscheduled DNA synthesis following excimer laser ablation of the cornea in vivo. Invest Ophthalmol Vis Sci. 1987;28(2):287-294.
- Trokel SL, Srinivasan R, Braren B. Excimer laser surgery of the cornea. Am J Ophthalmol. 1983;96(6):710-715.
- Peyman GA, Kuszak JR, Weckstrom K, Mannonen I, Viherkoski E, Auterinen L. Effects of XeCL excimer laser on the eyelid and anterior segment structures. Arch Ophthalmol. 1986;104(1):118-122.
- Puliafito CA, Wong K, Steinert RF. Quantitative and ultrastructural studies of excimer laser ablation of the cornea at 193 and 248 nanometers. Lasers Surg Med. 1987;7(2):155-159.
- Dehm EJ, Puliafito CA, Adler CM, Steinert RF. Corneal endothelial injury following excimer laser ablation and 193 and 248 nm. Arch Ophthalmol. 1986;104(9):1364-1368.
- Puliafito CA, Stern D, Krueger RR, Mandel ER. High-speed photography of the excimer laser ablation of the cornea. Arch Ophthalmol. 1987;105(9):1255-1259.
- Autrata R, Rehurek J, Vodickova K. Phototherapeutic keratectomy in children: 5-year results. J Cataract Refract Surg. 2004;30(9):1909-1916.
- O'Brart DP, Muir MG, Marshall J. Phototherapeutic keratectomy for recurrent corneal erosions. Eye (Lond). 1994;8(4):378-383.
- Baryla J, Pan YI, Hodge WG. Long-Term Efficacy of Phototherapeutic Keratectomy on Recurrent Corneal Erosion Syndrome. Cornea. 2006;25(10):1150-1152.
- Ohman L, Fagerholm P, Tengroth B. Treatment of recurrent corneal erosions with the excimer laser. Acta Ophthalmol. 1994;72(Pt 4):461-463.
- Fagerholm P, Fitzsimmons TD, Orndahl M, et al. Phototherapeutic keratectomy: Long-term results in 166 eyes. Refract Corneal Surg. 1993;9(2 Suppl):S76-S81.
- Dogru M, Katakami C, Miyashita M, et al. Ocular surface changes after excimer laser phototherapeutic keratectomy. Ophthalmology. 2000;107(6):1144-1152.
- Rosa N, Cennamo G. Phototherapeutic keratectomy for relief of pain in patients with pseudophakic bullous keratopathy. J Refract Surg. 2002;18(3):276-279.
- Koksal M, Kargi S, Gurelik G, Akata F. Phototherapeutic keratectomy in Schnyder crystalline corneal dystrophy. Cornea. 2004;23(3):311-313.
- Alio JL, Belda JI, Shalaby AM. Correction of irregular astigmatism with excimer laser assisted sodium hyaluronate. Ophthalmology. 2001;108(7):1246-1260.
- Maloney RK, Thompson V, Ghiselli G, Durrie D, Waring GO 3rd, O'Connell M. The Summit Phototherapeutic Keratectomy Study Group. A prospective multicenter trial of excimer laser phototherapeutic keratectomy for corneal vision loss. Am J Ophthalmol. 1996;122(2):149-160.
- Campos M, Nielsen S, Szerenyi K, Garbus JJ, McDonnell PJ. Clinical follow-up of phototherapeutic keratectomy for treatment of corneal opacities. Am J Ophthalmol. 1993;115(4):433-440.
- Ma JJ, Tseng SS, Yarascavitch BA. Anterior segment optical coherence tomography for transepithelial phototherapeutic keratectomy in central corneal stromal scarring. Cornea. 2009;28(8):927-929.
- Amm M, Duncker GI. Refractive changes after phototherapeutic keratectomy. J Cataract Refract Surg. 1997;23(6):839-844.
- Katsube N, Wang R, Okuma E, Roberts C. Biomechanical response of the cornea to phototherapeutic keratectomy when treated as a fluid-filled porous material. J Refract Surg. 2002;18(5):S593-S597.
- Dinh R, Rapuano CJ, Cohen EJ, Laibson PR. Recurrence of corneal dystrophy after excimer laser phototherapeutic keratectomy. Ophthalmology. 1999;106(8):1490-1497.
- Taenaka N, Fukuda M, Hibino T, et al. Surgical therapies for Acanthamoeba keratitis by phototherapeutic keratectomy and deep lamellar keratoplasty. Cornea. 2007;26(7):876-879.
- Abbas UL, Hersh PS; Summit PRK Study Group. Late natural history of corneal topography after excimer laser photorefractive keratectomy. Ophthalmology. 2001;108(5):953-959.
- Gartry DS, Larkin DF, Hill AR, Ficker LA, Steele AD. Retreatment for significant regression after excimer laser photorefractive keratectomy: A prospective, randomized, masked trial. Ophthalmology. 1998;105(1):131-141.
- Alkara N, Genth U, Seiler T. Diametral ablation--a technique to manage decentered photorefractive keratectomy for myopia. J Refract Surg. 1999;15(4):436-440.
- Rachid MD, Yoo SH, Azar DT. Phototherapeutic keratectomy for decentration and central islands after photorefractive keratectomy. Ophthalmology. 2001;108(3):545-552.
- Taneri S, Koch JM, Melki SA, et al. Mitomycin-C assisted photorefractive keratectomy in the treatment of buttonholed laser in situ keratomileusis flaps associated with epithelial ingrowth. J Cataract Refract Surg. 2005;31(10):2026-2030.
- Steinert RF, Ashrafzadeh A, Hersh PS. Results of phototherapeutic keratectomy in the management of flap striae after LASIK. Ophthalmology. 2004;111(4):740-746.