Authors: Loh-Shan Leung, MD; Mark S. Blumenkranz, MD
Thermal Laser Photocoagulation
Thermal photocoagulation with the argon blue-green laser was the first treatment for exudative age-related macular degeneration (AMD). The results were modest and typically benefited only patients with lesions outside the center of the capillary free zone (CFZ), which occupies the central 500 microns of the macula in the area of greatest acuity and cone density.
Initially described in the late 1960s, the argon laser was considered controversial for many years, and was demonstrated to be effective with the 1982 publication of the Macular Photocoagulation Study (MPS).In that study, which began in 1978, 224 patients with choroidal neovascularization no closer than 250 microns and extending up to 2,500 microns from the center were randomized to thermal photocoagulation with the argon laser or observation.
Treatment typically consisted of the application of contiguous 100 micron spots with durations of 200 ms to create an intense white burn. The study was terminated early when it was determined that twice as many eyes had a vision better than 20/40 at their most recent visit than those assigned to observation, and that only 25% of treated eyes experienced severe vision loss compared with 60% of observed eyes, based on cumulative proportions at 18 months.
After publication of those results, thermal photocoagulation became the standard of care for treatment of extrafoveal lesions. Additional patients were then studied in this trial, including those with juxtafoveal and subfoveal neovascularization. Initially patients in the juxtafoveal group showed improved outcomes compared with controls, but by 3 years, there was no significant difference between groups. Even in eyes with extrafoveal choroidal neovascularization, which were studied in the original trial, late recurrences typically occurred on the foveal side of the treatment became increasingly common with time and the long-term prognosis for preservation of good foveal function was poor (Figure 1). A final group presenting with subfoveal neovascularization was studied using thermal photocoagulation. Direct treatment to the lesions located in the center of the capillary free zone resulted in vision loss compared with untreated controls immediately following randomization to treatment, but over time were judged to have a superior anatomic and visual outcome than those untreated. The study led to the recommendation of treating these lesions with intense thermal burns, but many clinicians objected to this approach because of the immediate destruction of central vision in patients who otherwise had various degrees of residual macular function. This treatment was never widely adopted despite the recommendation.
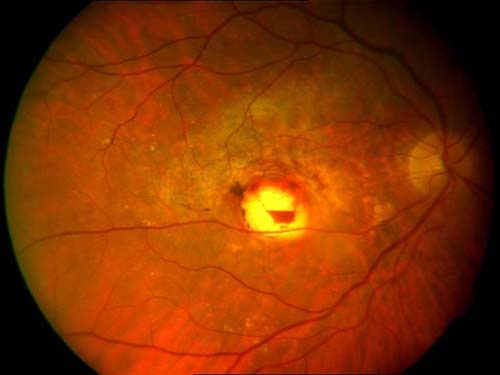
Figure 1. Juxtafoveal thermal laser for CNV: marginal recurrence of CNV on foveal edge.
Thermal photocoagulation was essentially abandoned for the treatment of any forms of exudative AMD. To reduce the potential complications associated with thermal laser, including visual field defects and the propensity for aggressive recurrence that eventually may involve the foveal center, most clinicians prefer to treat with photodynamic therapy (PDT). There has been some intermittent interest in the potential use of thermal laser for drusen, which has been shown to reduce the size and number of drusen, albeit at the risk of an increased frequency of what is presumed to be iatrogenic choroidal neovascularization. With the use of short pulse duration lasers and the creation of subvisible lesions, this form of treatment may yet prove to be a valid therapeutic option (Figure 2).
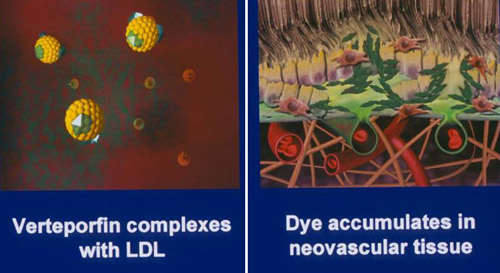
Figure 2. PDT with verteforfin: an early advance in treating subfoveal CNV.
Ocular Photodynamic Therapy
Ocular PDT was first described for the treatment of exudative AMD (“wet” AMD). A form of selective laser therapy, PDT has been demonstrated to lead to the closure of choroidal neovascular and other active proliferating vessels, while leaving normal retinal tissue unharmed. It was the first treatment to show efficacy in reducing the risk of moderate and severe vision loss in a disease that previously had an almost uniformly poor visual prognosis.
The principles of PDT were originally identified in the earliest part of the 20th century. The term was originally coined in German, Photodynamisagerscheinung, in 1904 by Tapeiner and Jodlebauer, as a reaction requiring light, a photosensitizing agent, and oxygen. Twenty years later, Policard recognized that some tumors gave off a red-orange fluorescence when stimulated by ultraviolet light, a reaction thought to originate from endogenous porphyrins released after tumor invasion by hemolytic bacteria. Initially advocated for oncology, PDT involves the systemic administration of a photosensitizing agent by intravenous injection, followed by local application of light in the absorption spectrum of that agent, causing closure of hyperproliferative vessels, as in an actively growing tumor, or in the case of ophthalmology, in an area of active choroidal neovascularization. PDT is best suited for areas easily accessible to light, and has frequently been used for cutaneous or subcutaneous tumors. Some of the first applications were for esophageal or endobronchial tumors where light was delivered by endoscope.
The eye is an ideal target for PDT, given the ease and specificity with which laser energy can be focused and delivered to diseased tissue. Prior to the development of verteporfin PDT, a related compound, hematoporphyrin derivative was described for the treatment of choroidal melanoma, and although PDT has largely been supplanted by pharmacologic agents with specific molecular targets in the treatment of AMD, it remains a useful adjuvant therapy for other intraocular vascular disorders, as well as posterior segment neoplasms.
A basic understanding of the so-called photodynamic reaction allows a mechanistic description of action of PDT. Upon absorption of a photon of light, a photosensitizer transitions from its stable, singlet ground state (its electrons have paired, opposite spin) to a transient excited singlet state (an electron moves to a higher energy excited level, but the electrons retain paired, opposite spin). This excited state may then either decay through fluorescence (immediate release of the photon and return of the electron to its ground state), or transition via intersystem crossing to an excited triplet state (in which the excited-state electron reverses its spin to give a spin quantum number of 1. This excited triplet state is the primary actor in the photodynamic reaction.
Mechanisms
There are two types of interactions the excited triplet state sensitizer has with biologic tissue:
- The type I process involves the direct interaction of the excited sensitizer with the target tissue, leading to energy transfer from the sensitizer to the substrate via electron or hydrogen atom.
- The type II process involves energy transfer to the ground state triplet oxygen (one of the few naturally occurring molecules that exist in the triplet state as its ground state), leading to the formation of excited singlet oxygen. Singlet oxygen species are highly oxidative, and transfer energy readily to surrounding tissue, damaging cellular components and leading to cell death. The type II reaction is more common in oxygen rich environments, with singlet oxygen believed to be the most significant cause of cellular damage.
Most photosensitizers used in PDT are of the porphyrin class, composed of tetrapyrrolic macrocycles that are ubiquitous biologic molecules found in nature. A reduction of one of the four pyrrole rings in the porphyrin macrocycle yields a chlorin molecule that alters the electronic conjugation system, increasing the absorption properties from 630 to approximately 660–690 nm. The predominant photosensitizing agent used in ophthalmology is benzoporphyrin derivative monoacid ring A (BPD-MA), or verteporfin (Visudyne). The “ring A” refers to the conjugation of a cyclohexadiene ring on the “A pyrrole” position of the chlorin structure. BPD-MA consists of equal amounts of two regioisomers that differ in the location of the carboxylic acid and methyl ester on the C and D rings of the chlorin macrocycle. Both monoacid regioisomers are converted to the diacid in the liver. (Superior photosensitizing efficiency over the diacid was the reason for developing the monoacid analogues.) In humans, the plasma half-life of BPD-MA is 5-6 hours. BPD-MA is hydrophobic and is therefore solubilized as a liposomal formulation.
The selectivity of PDT is due in part to its accumulation within microvasculature, and specifically its ability to cause damage to endothelial cells. Verteporfin has high affinity for plasma lipoproteins and is taken up by cells with many low-density lipoprotein receptors, such as neovascular endothelium. Laboratory evidence suggests that tumor death caused by PDT is a result of vascular damage and thrombosis, with subsequent tumor anoxia. For treatment of the retina, when drug dosing and light of the appropriate fluence are optimally applied, closure of the choriocapillaris and choroidal neovascular tissue can be achieved without damage to the overlying retinal tissue or vasculature. In contrast, traditional photocoagulation results in necrosis to the retinal pigment epithelium (RPE), the underlying choroidal tissue, as well as thermal conductance to the overlying retina. The selective, localized effect of PDT has significant potential benefit for visual function; whereas macular photocoagulation for AMD results in immediate, profound damage to the retina. PDT should preserve retinal anatomy (Figure 2).
Treatment Application
Treatment with PDT should be guided by recent fluorescein angiography. As determined by phase I-III clinical studies for AMD, verteporfin is typically administered via intravenous infusion at a dose of 6 mg/m2 over a period of 10 minutes. Nonthermal laser is then applied using a slit-lamp system. Fifteen minutes after the initiating the infusion, low intensity nonthermal light at a wavelength of 689 nm and with a power density or irradiance of 600 mW/cm2 is applied for an exposure duration of 83 seconds, corresponding to a total fluence of 50 J/cm2. An upper limit of 150 J/cm2 has been associated with retinal ischemia, while lower doses selectively cause localized choroidal vascular closure without widespread choroidal vascular dysfunction.
A light dose of 50 J/cm2 is so-called “full-fluence” PDT; half-fluence PDT (25 J/cm2—the minimal level required for closure of choroidal vessels) has been described for the treatment of choroidal neovascularization of various conditions, in addition to other choroidal vascular pathologies, including chronic central serous choroidopathy, polypoidal choroidal vasculopathy, as well as choroidal neoplasms, such as circumscribed choroidal hemangioma (Figure 3).
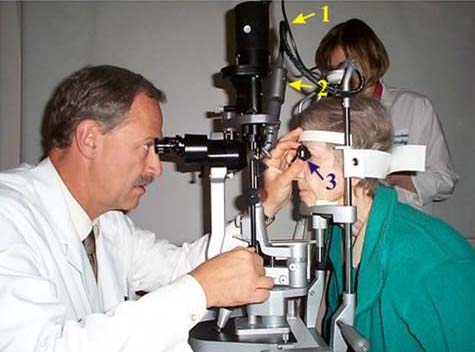
Figure 3. Laser photocoagulation on a slit-lamp system: 1. Cable connecting laser to slit-lamp. 2. Optical coupler projecting beam from fiber onto retina. 3. Contact lens.
PDT Clinical Trials
The use of PDT for exudative AMD has been rigorously tested in randomized, double-masked, phase III clinical trials. The Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) study examined 609 eyes across 22 centers in North America and Europe with choroidal neovascularization resulting from AMD. According to study design, patients were randomized 2:1, with 402 patients receiving verteporfin PDT and 207 patients receiving placebo. Inclusion criteria included a lesion component of classic CNV. Patients were treated as frequently as every 3 months, with the primary endpoint being the loss of less than 15 letters of vision. After 12 months, 61% of patients receiving PDT achieved this endpoint, versus 46% of those in the placebo group (p<0.001), with the difference persisting after 2 years (53% versus 38%, p<0.001). Additionally, a greater number of patients in the PDT group lost less than 30 letters of vision (82 versus 70%); treatment benefits at both 1 and 2 years were greatest for lesions that were predominantly classic (classic component made up more than 50% of the total lesion size). However, there remained a mean net loss of letters in both treatment and placebo groups.
The Verteporfin in Photodynamic Therapy (VIP) trial examined certain patients who were excluded or not specifically studied in the TAP trial: patients with progressive occult neovascular membranes without a classic component; patients with classic neovascularization and good vision (better than 20/40); and patients with choroidal neovascularization deemed secondary to pathologic myopia. All patients in the occult CNV and pathologic myopia arms of the study had vision better than 20/100. There were 339 patients with AMD enrolled in the study; most of the patients had occult neovascularization with no classic component. Retreatment criteria were identical to those in the TAP trial. At 1 year, the number of patients losing less than 15 letters of vision was similar; however, after two years, 54% in the PDT had suffered this moderate level of vision loss, compared with 67% in the placebo group (p=0.02). Moreover, only 30% of patients receiving PDT suffered 30 letters or more of vision loss, compared with 47% of those receiving placebo (p=0.001). For patients with only occult disease, the results were similar, with 55% (PDT) versus 68% (placebo) losing 15 or more letters (p=0.03), and 29% (PDT) vs. 47% (placebo) losing 30 or more letters (p=0.004) at 24 months. There was insufficient power to perform a subgroup analysis for patients with classic CNV only. The myopia arm of the VIP study included 81 patients, with 42 receiving PDT. In this subset of patients, 72% of treated patients lost less than 8 letters of vision, compared with 44% of those receiving placebo (p<0.01) (Figure 4). The extension of treatment effect on closure of choridal neovascularization may be further extended by the simultaneous application of intravitreal triamcinolone at treatment sessions immediately following the application of the laser activation (Figure 5).
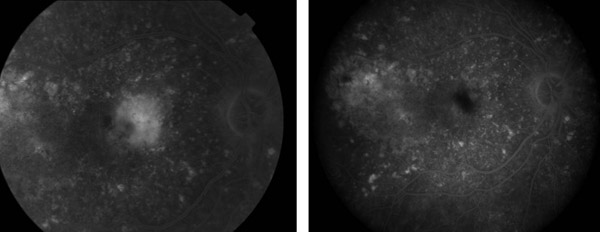
Figure 4. Pre- and post-treatment FA: Vision 20/30 six months post-PDT for exudative AMD.
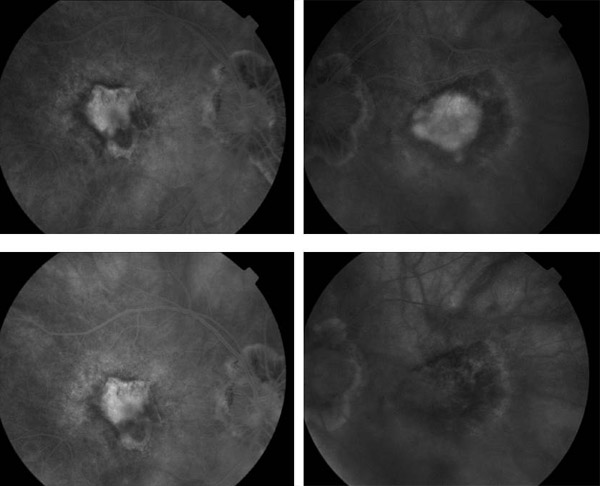
Figure 5. Pre- and post-treatment FA: IVFA stable at 1 month (left) and 3 months (right).
The most common adverse reactions associated with PDT include visual disturbances and infusion site reactions. A small percentage of patients report sudden decrease in vision following treatment. Less commonly reported events include infusion related back pain and vitreous hemorrhage. Photosensitivity reactions have been reported as well, characterized as a sunburn, usually occurring within 24 hours of verteporfin infusion. Rarely reported events include choroidal nonperfusion and RPE tears. Patients are typically advised to use protective clothing and avoid sun exposure when possible.
As vascular endothelial growth factor (VEGF) blockade has become the first-line treatment for exudative AMD, PDT is used less frequently. However, there remain select indications for PDT advocated by retina specialists, including patients not candidates of anti-VEGF therapy and those refractory to anti-VEGF therapy.
PDT for Other Indications
Polypoidal choroidal vasculopathy (PCV) is a relatively uncommon condition, often with similar clinical features to those of AMD. Originally believed to have a racial and gender predilection for women of African extraction, it was later determined to be common in a number of non-Caucasian races, and is especially prevalent in Asian populations. It was initially termed posterior uveal bleeding syndrome due to the frequent presence of subretinal and sub-RPE hemorrhage.
Indocyanine green (ICG) angiography is the primary modality used in making the diagnosis of PCV. After injection of ICG dye, PCV is characterized by the appearance of hyperfluorescent polyp-like structures within the choroidal circulation. There may also be a visible choroidal branching vascular network. Polyps may be clinically evident as dilated reddish-orange nodules. Although PCV often has a favorable natural history when compared with neovascular AMD, the occurrence of devastating subretinal bleeding can lead to significant visual disability. When PCV results in recurrent subfoveal bleeding, cystoid macular edema, or subretinal fluid, treatment may be indicated.
PCV often responds readily to both full-fluence and reduced-fluence PDT. Protocol typically dictates treatment of the area encompassing the entire area of polyps, the branching vascular network, if seen, and an additional margin of 1000 mm beyond the greatest linear dimension of the lesion. Long-term, successful outcomes of up to 5 years after initiating treatment have been reported. Visual outcomes are usually good, with up to 100% of patients experiencing visual stability or improvement. Additionally, up to 95% of patients have resolution of polyps, although repeat treatments are required in a majority of patients, likely due to the persistence of the branching vascular network. Studies have reported up to 2.2 injections required over a 5-year period, with shorter term studies suggesting that the majority of treatments are performed in the first 1-2 years.
Following a number of case reports and series of successful treatment of PCV with combined therapy using an anti-VEGF agent with PDT, the EVEREST study was a randomized, controlled, double-masked trial comparing the efficacy (by anatomic criteria) of PDT monotherapy, PDT combination therapy with ranibizumab, and ranibizumab monotherapy. Sixty-one Asian patients were randomized to 1 of the 3 treatments, with combination therapy or PDT monotherapy resulting in superior anatomic outcomes (resolution of polyps) at 6 months. Visual outcomes were not statistically significant among the 3 groups, with all gaining vision.
The most commonly reported adverse events specific to PDT treatment of PCV are subretinal hemorrhage, and occasionally vitreous hemorrhage requiring vitrectomy, although the incidence of these events is rare.
Central serous choroidopathy (CSC) is believed to be a disorder of abnormal choroidal permeability, possibly combined with RPE homeostasis resulting in fluid accumulation under the neurosensory retina and RPE. Classically affecting young men, the disease has been associated with systemic corticosteroid use, “type A” personality traits, and life stressors. In the vast majority of patients, CSC resolves spontaneously with no intervention necessary, and visual outcomes are usually good. However, chronic subretinal fluid may persist in a minority of patients, ultimately leading to photoreceptor degeneration and outer retinal and retinal pigment epithelial atrophy. When present in the fovea, this may result in significant visual impairment.
In select cases, acute or chronic CSC with focal leakage on fluorescein angiography can be treated with low intensity thermal laser. However, in cases with subfoveal or juxtafoveal leakage, or with diffuse leakage, traditional laser photocoagulation is more problematic. With ICG angiographic guidance, PDT has proven to be an effective option treatment for both acute and chronic CSC. Standard PDT (6 mg/m2 verteporfin, 50 J/cm2) has been reported to successfully treat chronic CSC in 60-100% of cases, in series of up to 82 patients. However, after PDT for CSC, progressive RPE atrophy, choroidal ischemia, choroidal neovascularization, and RPE tears have been reported. Several authors have proposed that reduced-fluence or reduced dose PDT may decrease these risks. One study has shown reduced rates of choriocapillaris ischemia/nonperfusion and retinal atrophy in half-fluence PDT as compared to standard PDT, with similar visual outcomes and rates of fluid resolution (Figure 6).
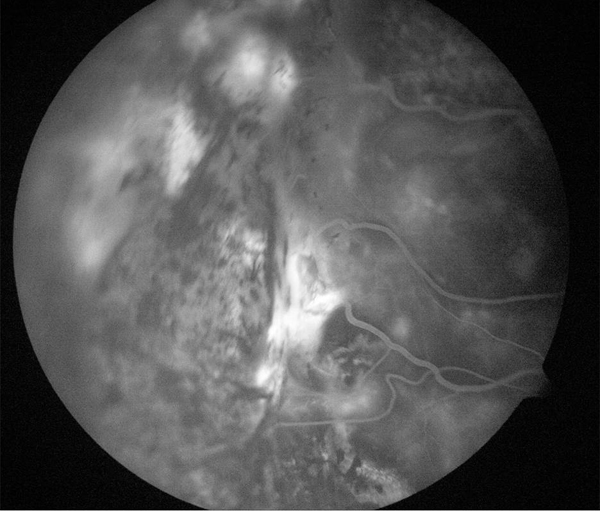

Figure 6. Treatment of leaking angioma from VHL treated with laser.
Currently, standard fluence PDT, reduced-fluence PDT, or reduced-dose PDT may be considered for the treatment of CSC, aided by indocyanine green (ICG) angiography. As with exudative AMD, the treatment area typically encompasses a 1-mm margin beyond the greatest linear dimension of the lesion. Disease activity may be followed using ICG angiography (decreased leakage) or with OCT (reduction in subretinal fluid, as well as decreased choroidal thickness on enhanced depth imaging).
Suggested Reading
- Macular Photocoagulation Study Group. Argon laser photocoagulation for senile macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1982;100(6):912-918.
- Macular Photocoagulation Study Group. Laser photocoagulation of juxtafoveal choroidal neovascularization. Five-year results from randomized clinical trials. Arch Ophthalmol. 1994;112(4);500-509.
- Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol.1991;109(9):1232-1241.
- Complications of Age-related Macular Degeneration Prevention Trial (CAPT) Research Group. Risk factors for choroidal neovascularization and geographic atrophy in the complications of age-related macular degeneration prevention trial. Ophthalmology. 2008;115(9):1474-1479.